Spectrophotometric Determination of Manganese by Biologically Active 2-Hydroxy-4-Methoxy Acetophenone Oxime
F. Rehman and Samya Mairaj
Department of Analytical Chemistry, Faiz-E-Am Degree College, Meerut (India).
2-Hydroxy-4-methoxy acetophenone oxime (HMAO) has been successfully employed as a reagent for spectrophotometric determination for Mn(II) at pH range 8.0 to 11.0 in chloroform medium. The composition of the complex 1:2 (metal:ligand) has been confirm by job’s method for continuous variation, Yoe & jones mole ratio method & slope ratio method. The stability constant of the complex is found to be 5.648x107. The dark brown coloured complex obeys Beer’s law over the concentration range1 to 12 ppm for Mn(II) ion. The complex has molar absorptivity 2.40 x 102 mol-1cm-1. While their sensitivity is 0.228 ìg Mn/cm2.Limits of interference due to the presence of foreign ions in the spectrophotometric determination has also been determined. The standard free energy of the complex is 1.06 Kcal/mole at 30°C. The complex is stable for one week, HMAO has also been found to give quite satisfactory results for Mn(II) present in synthetic mixtures. The antimicrobial activity of HMAO and Mn-HMAO complex have also been calculated.
KEYWORDS:Manganese; Acetophenone Oxime; HMAO; Ions
Download this article as:| Copy the following to cite this article: Rehman F, Mairaj S. Spectrophotometric Determination of Manganese by Biologically Active 2-Hydroxy-4-Methoxy Acetophenone Oxime. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Rehman F, Mairaj S. Spectrophotometric Determination of Manganese by Biologically Active 2-Hydroxy-4-Methoxy Acetophenone Oxime. Available from: http://www.orientjchem.org/?p=23459 |
Introduction
A number of reagents are known for spectrophotometric and complexometric determination of Mn(II) and other transition metal ions1-5. The present communication deals with the spectrophotometric determination of Mn(II) with HMAO & compare their biological behaviour with standard drug.
Experimental
Standard stock solution (0.1M) of Mn(II) has been prepared by dissolving appropriate amount of MnCl2 (AR) in double distilled water. The amount of Mn(II) in stock solution was determined by standard method.6
Preparation of 2-Hydroxy-4-methoxy acetophenone oxime ( HMAO)
2,4-Dihydroxy acetophenone(resacetophenone) and 2-hydroxy-4-methoxy acetophenone were prepared by standard methods 7,8 Oxime of HMA has been prepared by refluxing HMA with hydroxyl amine hydrochloride in the presence of sodium acetate in ethanol medium for 2 hrs. The product separated on concentration and subsequent dilution and recrystallised from ethanol in to colourless needle like crystals (mp101◦С)with m.w. 181.24 gm ( calcd for C9H11NO3 ).The structure of the compound was confirm by elemental analysis. IR spectra, NMR spectra and Mass spectra.
Preparation of Mn(II) –HMAO complex and selection of solvent
When metal ion solution was treated with an ethanolic solution of HMAO and the mixture was stirred for about an hour at room temperature, dark brown precipitates of complex were obtained in the pH range 8.0-11.0. The complex was found to be insoluble
in polar solvents like water, methanol or ethanol but soluble in non polar solvents like chloroform, benzene, CCl4 etc. This complex was more soluble in chloroform so it was elected as a solvent for extractive spectrophotometric determination of Mn(II).
Apparatus
Systronics UV/VIS spectrophotometer (model-118) was used for absorbance measurement & systronic pH meter (model 335) for pH measurement. .
Results and Discussion
Selection of optimum wavelength & pH
The formation of Mn(II) complex with HMAO & their stability are depend on the pH of solution. While absorbance is depend upon the wavelength.
The method of Vosburg & cooper showed the formation of only one complex having λmax at 410 nm.The absorbance of complex formation was measured at room temperature (300 K) at regular intervals of time up to two week & also different temperature varies from 300K to 325K. The results show that complex is stable for one week & upto 318K without change of absorbance.
The Mn(II) complex exhibit maximum & almost constant absorbance in the pH range 8.0 to 11.0.( Table-2). The subsequent studies were, therefore, carried out at pH 10.0. It was also found that a 16 fold excess of the reagent was necessary to attain the maximum colour intensity.
Table 1: VOSBURG AND COOPER’S METHOD FOR Mn(II) AND HMAO
Concentration of Mn(II) = HMAO = 1.0 x 10 -2 M
Total Volume = 16.0 ml
| Wave length Ratio of Metal to ligand |
| (nm) 1:1 OD 1:2 OD 1:3 OD 1:4 OD |
| 410 0.580 0.785 0.785 0.330 |
| 420 0.540 0.750 0.780 0.290 |
| 430 0.450 0.670 0.750 0.230 |
| 440 0.365 0.535 0.690 0.185 |
| 450 0.315 0.410 0.600 0.150 |
| 460 0.280 0.340 0.500 0.120 |
| 480 0.215 0.270 0.390 0.100 |
| 500 0.150 0.220 0.295 .080 |
| 520 0.110 0.190 0.240 .060 |
| 540 0.100 0.150 0.210 .040 |
| 560 .090 0.140 0.200 .030 |
| 580 .085 0.135 0.200 .030 |
| Metal (ml) 6.0 4.0 3.0 8.0 |
| Ligand (ml) 6.0 8.0 9.0 4.0 |
| Fig. 4.33 A B C D |
Table 2: EFFECT OF pH ON THE ABSORBANCE OF Mn(II)-HMAO AT 410 nm
Concentration of Mn(II)=HMAO = 1.0 x 10-3 M
Volume of Mn(II) Solution = 2.0 ml
Volume of HMAO Solution = 4.0 ml
Total Volume = 8.0 ml
| pH | O.D | pH | O.D |
| 7.0 | 0.080 | 9.5 | 0.250 |
| 8.0 | 0.120 | 10.0 | 0.280 |
| 8.5 | 0.180 | 10.5 | 0.280 |
| 9.0 | 0.210 | 11.0 | 0.280 |
Reproducibility
Absorbance measurements of a set of six solution prepared in a similar way & have the same concentration of all the reagents show that the reproducibility of measurements are quite good with standard deviation ± 0.432 i.e. 0.26 %.
Stoichiometry and stability constant of the complex
Results of yoe and jones mole ratio method9, slope ratio method10 and job’s method of continuous variation11 establish the formation of 1:2 ( metal: ligand) complex.(Fig 2,3)
The stability constant of the complex was calculated from the following relationship using mole ratio method. (Table-3)
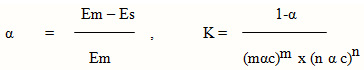
Came to be 5.468×107. The value of the standard free energy of the formation of complex from the expression ∆ G = -2.303 RT log K , came to be 1.06 Kcal/ mol at 30◦C.
Table 3:COMPOSITION OF THE Mn(II)- HMAO COMPLEX BY MOLE RATIO METHOD AT410 nm AND pH 10.0
Concentration of Mn(II) Solution = HMAO = 0.5 x 10-3 M
Constant Volume of Mn(II) = 2.0 ml
Total Volume = 16.0 ml
Ionic strength = 0.1 M NaClO4
| Volume of HMAO | Volume of HMAO | ||
| Solution (ml) | OD | Solution (ml) | OD |
| 1.0 | 0.175 | 6.0 | 0.278 |
| 2.0 | 0.225 | 7.0 | 0.298 |
| 3.0 | 0.245 | 8.0 | 0.298 |
| 4.0 | 0.254 | 9.0 | 0.298 |
| 5.0 | 0.257 |
Validity of Beer’s law and optimum concentration range
It was observed that the Beer’s law is obeyed in the concentration range 1-12 ppm of Mn(II). The optimum concentration range for determination of Mn(II) in solution as deduced from Ringbom plot 12. From the slope ratio curve, the molecular extinction coefficient of the complex is 2.40×102 while the value of photometric sensitivity as per sendell’s scale is found to be 0.228 μg-Mn/cm2
Effect of foreign ions
The effect of foreign ion on the spectrophotometric determination of manganese was studied by adding different ions quantitatively in a stock solution of Mn(II) ion. Mn(II) was extracted as Mn(II)-HMAO complex in the usual manner after adjusting the pH of solution at 10.0. It was observed that at 9 ppm of Mn(II),1500 ppm of Cl-,SO42- &CH3COO-,1200 ppm of NH4+, K+,Na+, NO3-,Br- and I-: 800 ppm of SO32- & NO2-, 500 ppm of Ca2+, Ba2+,Sr2+, tartarate & citrate; 150 ppm of Zn2+,Cd2+ and Be2+ could be tolerated. However Cu2+, Pd2+, Co2+, Fe3+ & UO22+ interfered seriously.
The precision & accuracy of spectrophotometric method were tested by analysing sample containing the known amount of Mn(II). It was found that the standard deviation of the method does not exceed 2 % & the accuracy of the procedure in limits accepted for spectrophotometric determinations.
Determination of Manganese from different samples
The usefulness of the reagent in estimation of manganese was determined from various samples of Mn(II) concentration having different concentration. The sample mixture containing manganese metal were taken for spectrophotometric analysis at different wavelength. The result are given in table (IV)
Table-4: Analysis of Manganese in various sample
| Sample | Mn taken | Mn found | Absorbance | Relative error |
| μg | μg | |||
| Synthetic Mixture No.1 | 150 | 0.420
0.410 0.180 Avg. |
164.5
159.3 167.4 163.7 |
0.13 |
| Synthetic Mixture No.2 | 120 | 0.310
0.326 0.334 Avg. |
112.6
120.1 123.4 118.7 |
0.91 |
| Synthetic Mixture No.3 | 206 | 0.549
0.547 0.552 Avg. |
202.3
209.6 211.6 207.8 |
0.87 |
Analysis
The extracted complex was concentrate & crystallized in desiccator. The elemental analysis of the complex shows metal:ligand (M:L) ratio to be 1:2
Founed C 53.21 H 4.93 H 7.26 Mn 12.75
Calculated C 53 H 4.82 H 7.48 Mn 12.99
The IR spectra of HMAO and complex revealed that the –OH ( Stretch) band at 3280
cm-1 for the HMAO disappears during complexation .i.e. complex formation takes place through the N of oximino group and oxygen of the hydroxyl group.
The general composition of the complex is [ C18H20N2O6Mn ]
Antimicrobial activity
HMAO & Mn-HMAO were screened for their antibacterial activity against E.Coli & antifungal activity against Aspergillus niger using agar diffusion 13 method & dry weight increased method14. The results indicate that HMAO & its Mn(II) complex exhibit more antimicrobial activity with respect to standard drug.
Mode of action
Antimicrobes exert their action in the different ways
Inhibition of cell wall synthesis.
Inhibition of cell membrane
Inhibition of Biosynthesis ( i.e. production of purines,pyrimidine,Amino acid, Vitamins, Protien, RNA, DNA)
Inhibitors of energy production ( inhibit the respiration or by uncoupling of oxidative phosphorylation ( Disruption the metabolic activities.)
The studies demonstrated that chelation can increase antimicrobial activities than ligand. It has been suggested that metal chelation reduced polarity of metal ion 15 mainly because of the partial shairing of its positive charge with the donor group and possibility of the d-electron delocalization occurring with in the whole chelate ring system formed during coordination. This process of chelation thus increase the lipophilic nature of the central metal atom which in turn favours its permeation through the lipoid layer of the membrane.16

This work is licensed under a Creative Commons Attribution 4.0 International License.









