Simple and Efficient One Step Synthesis of Functionalized Flavanones and Chalcones
Abdullah Saad Albogami, Usama Karama, Ahmed Amine Mousa, M. Khan, Sara Abdullah Al-Mazroa and Hamad Z. Alkhathlan*
Department of Chemistry, Faculty of Science, King Abdulaziz University, North Jeddah (Saudi Arabia).
A facile and highly efficient microwave-assisted synthesis of functionalized chalcones and flavanones based on the Claisen-Schmidt condensation reaction is reported. The method describes the synthesis of flavanones in single step with excellent yield and it was revealed that position and number of substituents on acetophenones and aromatic aldehydes played a very crucial and key role in the construction of flavanone derivatives. Among the thirty two synthesized compounds, five chalcones and one flavanone were novel compounds.
KEYWORDS:Flavanones; 2'-Hydroxyachalcones; Microwave Irradiation; Claisen-Schmidt Condensation
Download this article as:| Copy the following to cite this article: Albogami A. S, Karama U, Mousa A. A, Khan M, Al-Mazroa S. A, Alkhathlan* H. Z. Simple and Efficient One Step Synthesis of Functionalized Flavanones and Chalcones. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Albogami A. S, Karama U, Mousa A. A, Khan M, Al-Mazroa S. A, Alkhathlan* H. Z. Simple and Efficient One Step Synthesis of Functionalized Flavanones and Chalcones. Available from: http://www.orientjchem.org/?p=11816 |
Introduction
Chalcones and flavanones, originally isolated from natural sources are an outstanding class of naturally occurring bioactive compounds with a 1,3-diarylpropane skeleton belonging to the flavonoid family. Chalcones (1,3-diphenyl-2-propene-1-ones) either natural or synthetic have been reported to posses varieties of biological activities[1] including anticancer,2 antimitotic,3 antiinflammatory,4 antimalarial,5 antiangiogenic,6 antiinfective,4j antimicrobial, 7 antioxidative,2f-g,8 and antiproliferative9 activities. Similarly, flavanones (2,3-dihydro-2-phenylchromene-4-one) have also been reported to posses anticancer,10 antimicrobial,11 antiproliferative,9b anti-inflammatory,10a cardiovascular,10a antimalarial,5c,12 antiangiogenic,6 and hypotensive11b activities. These compounds have attracted significant interest of chemists, biochemists and pharmacologists due to their ample range of pharmacological activities and their uses as intermediates in the synthesis and biosynthesis of various classes of bioactive compounds.
Several methods have been reported for the synthesis of 2′-hydroxychalcones13 and flavanones13a,13c,13g,14. However, these methods are associated with several drawbacks such as long reaction time, poor yield and involvement of expensive catalysts. Furthermore, flavanones are always synthesized in two steps; in first step, 2′-hydroxychalcones are prepared via the most commonly used method, Claisen-Schmidt condensation reaction between 2-hydroxyacetophenones and benzaldehydes in the presence of aqueous alkaline bases. Second step involves subsequent cyclization of 2′-hydroxychalcones intermediate to form flavanones13a,13c,14. Nevertheless, conversion of chalcones into flavanones never completes and gives mixture of products. Also, during 2′-hydroxychalcones synthesis either the reaction never complete to give 2′-hydroxychalcones in high yield or part of 2′-hydroxychalcones cyclised to flavanone and give mixtures of chalocone and flavanone leading to tedious and time consuming column chromatographic separations. In spite of several drawbacks associated with chalcones and flavanones synthetic methods, to date, we could not find any previous report which describes the flavanones synthesis in one step. Moreover, in the last two decades use of microwave energy for conducting organic reactions has become a very popular and emerging technique as it has several advantages over classical organic reactions such as shortening reaction times, improving yields and promoting environmental friendly (Green chemistry) new reactions.
Experimental
Melting points were determined on a Gallen Kamp melting point apparatus and are uncorrected. Panasonic Microwave with model NN-C2003S/NN-C2002W was used for conducting reactions. 1H and 13C NMR spectra were recorded with a JEOL ECP-400 spectrophotometer. The NMR samples were prepared in CDCl3 with tetramethylsilane (TMS) as an internal standard. The chemical shifts and coupling constants (J) were expressed in d and Hz, respectively. MS spectra were recorded on Shimadzu QP5050A GC/MS system. The thin layer chromatography (TLC) was carried out on pre-coated silica gel 60 F254 (0.2 mm, Merck) plates. The developed TLC plates were visualized under UV light at 254 or 365 nm.
General Procedure for the Preparation of Flavanone and Chalcone Derivatives
Synthesis of flavanone and chalcone derivatives were carried out simply by mixing the appropriate amount of 2-hydroxyacetophenone derivatives (2.69 mmol) and aromatic aldehydes (2.69 mmol) in the presence of catalytic amount of aqueous KOH in MeOH and irradiating in a microwave oven at 100 W for 2 minutes. The reaction mixture was cooled, poured into crushed ice and then conc. HCl (1 ml) was added. The mixture was left to stay at 2-3°C overnight and the separated solid was collected by filtration, washed with water and recrystallized from methanol to give the desired products.
Results and Discussion
Previously, we reported a highly efficient synthesis of imines under microwave irradiotion15 and in continuation of our interest in the synthesis of heterocyclic compounds,16 we report here a simple and efficient microwave assisted synthesis of 2′-hydroxychalcones and flavanones in one step (Scheme 1) with high yield and no side products.
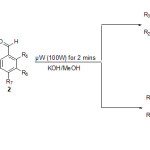 |
Scheme 1: MW-assisted synthesis of flavanones and chalcones |
The synthesis was carried out simply by mixing the appropriate amount of 2-hydroxyacetophenone derivatives and aromatic aldehydes in the presence of catalytic amount of aqueous KOH in MeOH and irradiating in a microwave oven at 100 W for 2 minutes to give the desired products. Two different types of compounds were obtained using the same reaction conditions; the first one is 2′-hydroxychalcones, 3a-3v, (Table 1), obtained when (i) R3 of the 2-hydroxyacetophenones, 1, = H, and the aldehydes, 2, have any type of substituents (ii) R3 ≠ H and there is more than one substituent on the aldehydes ring. The second type is flavanones, 4a-4j, (Table 2), obtained when R3 ≠ H and the aromatic aldehydes have either one substituent or less.
Table 1: Chalcone derivatives
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Product |
Yielda (%) |
|
H |
H |
H |
H |
H |
H |
H |
H |
3a |
81 |
|
H |
OMe |
H |
H |
H |
H |
H |
H |
3b |
84 |
|
H |
H |
H |
OMe |
H |
H |
H |
H |
3c |
80 |
|
H |
H |
H |
H |
H |
H |
Cl |
H |
3d |
90 |
|
H |
OMe |
H |
H |
H |
H |
Cl |
H |
3e |
92 |
|
H |
H |
H |
OMe |
H |
H |
Cl |
H |
3f |
81 |
|
H |
H |
H |
H |
H |
OMe |
OMe |
OMe |
3g |
91 |
|
H |
H |
OMe |
H |
H |
OMe |
OMe |
OMe |
3h |
89 |
|
H |
H |
CH3 |
H |
H |
OMe |
OMe |
OMe |
3i |
83 |
|
H |
H |
Cl |
H |
H |
OMe |
OMe |
OMe |
3j |
86 |
|
Cl |
H |
Cl |
H |
H |
OMe |
OMe |
OMe |
3k |
92 |
|
H |
H |
Br |
H |
H |
OMe |
OMe |
OMe |
3l |
84 |
|
H |
OMe |
H |
H |
H |
OMe |
OMe |
OMe |
3m |
90 |
|
H |
H |
H |
OMe |
H |
OMe |
OMe |
OMe |
3n |
88 |
|
H |
H |
H |
H |
Cl |
H |
Cl |
H |
3o |
82 |
|
H |
H |
OMe |
H |
Cl |
H |
Cl |
H |
3p |
89 |
|
H |
H |
CH3 |
H |
Cl |
H |
Cl |
H |
3q |
85 |
|
H |
H |
Cl |
H |
Cl |
H |
Cl |
H |
3r |
91 |
|
Cl |
H |
Cl |
H |
Cl |
H |
Cl |
H |
3s |
93 |
|
H |
H |
Br |
H |
Cl |
H |
Cl |
H |
3t |
80 |
|
H |
OMe |
H |
H |
Cl |
H |
Cl |
H |
3u |
94 |
|
H |
H |
H |
OMe |
Cl |
H |
Cl |
H |
3v |
87 |
Table 2: Flavanone derivatives
|
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
R7 |
R8 |
Product |
Yielda (%) |
|
H |
H |
Cl |
H |
H |
H |
H |
H |
4a |
85 |
|
H |
H |
OMe |
H |
H |
H |
H |
H |
4b |
81 |
|
H |
H |
CH3 |
H |
H |
H |
H |
H |
4c |
88 |
|
Cl |
H |
Cl |
H |
H |
H |
H |
H |
4d |
94 |
|
H |
H |
Br |
H |
H |
H |
H |
H |
4e |
92 |
|
H |
H |
Cl |
H |
H |
H |
Cl |
H |
4f |
92 |
|
H |
H |
CH3 |
H |
H |
H |
Cl |
H |
4g |
90 |
|
Cl |
H |
Cl |
H |
H |
H |
Cl |
H |
4h |
84 |
|
H |
H |
OMe |
H |
H |
H |
Cl |
H |
4i |
88 |
|
H |
H |
Br |
H |
H |
H |
Cl |
H |
4j |
89 |
a Isolated yield.
This result reveals that substitution at position 5 of the 2-hydroxyacetophenones, 1, plays an important and key role in the synthesis of flavanones. When 2-hydroxyacetophenone, 1, is substituted at position 5 with OCH3, CH3, Cl or Br and reacted with aromatic aldehydes having either one substituent or less, it gives flavanones in one step with very good yield ranging from 81-94%. On the other hand, when position 5 of 2-hydroxyacetophenone, 1, is not substituted and reacted with any type of aromatic aldehydes, it gives chalcones with yield ranging from 80-94%. To establish the general behavior of the reaction under aforedescribed conditions, twenty two representative 2′-hydroxychalcones, 3a-3v, and ten flavanones, 4a-4j, were prepared (Tables 1 and 2) among them five chalcones, 3n, 3p, 3q, 3t and 3v and one flavanone, 4h, were found to be novel compounds.
Conclusion
In conclusion, we have developed a convenient, rapid and highly efficient one step procedure for the preparation of functionalized flavanones and chalcones under microwave irradiation. On comparing the earlier reported methods for the preparation of flavanones13a,13c,14 with ours, it was found that this is a first report of its kind in which flavanones are being prepared in one step with very high yield.
2′-Hydroxychalcone (3a)
Yellow powder; mp 79oC (lit.[13h] mp 81-83oC); 1H and 13C NMR data is in agreement with Lit[13h]. MS: m/z (%) 224 (M+, 100), 147, 121, 103, 89, 77, 65, 51.
2′-Hydroxy-4′-methoxychalcone (3b)
Yellow powder; mp 97-98oC (lit.[8] mp 107-108oC); 1H NMR data is in agreement with Lit[8]. 13C NMR: d 56.2, 101.6, 108.3, 114.6, 120.9, 129.1, 129.6, 131.2, 131.8, 135.3, 145.0, 166.8, 167.3, 192.4. MS: m/z (%) 254 (M+, 100), 177, 165, 151, 122, 103, 77, 51.
2′-Hydroxy-6′-methoxychalcone (3c)
Yellow powder; mp 55oC (lit.[8] mp 65.0-65.5oC); 1H NMR data is in agreement with Lit[8]. 13C NMR: d 55.5, 101.1, 110.5, 111.5, 127.1, 128.0, 128.5, 129.9, 134.9, 135.5, 142.5, 160.6, 164.4, 194.1. MS: m/z (%) 254 (M+, 75), 177, 150, 103, 91, 77, 51.
4-Chloro-2′-hydroxychalcone (3d)
Yellow powder, mp 144-146oC (lit.[13i] mp 153-156oC); 1H and 13C NMR data is in agreement with Lit[13i]. MS: m/z (%) 258 (M+, 100), 238, 165, 147, 120, 101, 78, 65, 51.
4-Chloro-2′-hydroxy-4′-methoxychalcone (3e)
Yellow powder; mp 115-116oC; 1H NMR: 3.9 (s, 3H), 6.5 (d, J = 8.8 Hz, 2H), 7.4-7.6 (m, 5H), 7.8 (d, J = 15.5 Hz, 1H), 7.9 (d, J = 15.5 Hz, 1H), 13.4 (s, 1H). 13C NMR: d 55.7, 101.2, 107.9, 114.1, 120.9, 129.4, 129.8, 131.3, 133.4, 136.7, 143.0, 166.4, 166.9, 191.6. MS
289 (M++1, 43), 177, 151, 137, 122, 101, 75, 51
4-Chloro-2′-hydroxy-6′-methoxychalcone (3f)
Yellow powder; mp 114-116oC; 1H NMR: 3.9 (s, 3H), 6.4 (d, J = 8.0 Hz, 1H), 6.6 (d, J = 8.0 Hz, 1H), 7.4-7.6 (m, 5H), 7.8 (d, J = 15.4 Hz, 1H), 7.9 (d, J = 15.4 Hz, 1H), 13.1 (s, 1H). 13C NMR: d 56.1, 101.6, 111.1, 112.0, 128.1, 129.3, 129.7, 133.9, 136.2, 136.2, 141.5, 161.1, 165.0, 194.3. MS: m/z (%) 288 (M+, 100), 177, 150, 136, 122, 102, 75, 51.
2′-Hydroxy-3,4,5-trimethoxychalcone (3g)
Red needles; mp 146-148oC (lit.[7a] mp 180-182oC); 1H NMR data is in agreement with Lit[7a]. 13C NMR: d 56.3, 61.1, 106.0, 118.7, 118.9, 119.3, 129.7, 130.1, 136.5, 140.9, 145.7, 153.6, 163.7, 193.6. MS: m/z (%) 314 (M+, 98), 299, 283, 194, 181, 168, 147, 121, 93, 65.
2′-Hydroxy-3,4,5,5′-tetramethoxychalcone (3h)
Red needles; mp 115-117oC; 1H NMR: 3.7 (s, 3H), 3.8 (s, 3H), 3.9 (s, 6H), 7.0 (d, J = 8.8 Hz, 1H), 7.2 (m, 3H), 7.7 (d, J = 2.2 Hz, 1H), 7.8 (d, J = 15.4 Hz, 1H), 8.0 (d, J = 15.4 Hz, 1H), 12.1 (s, 1H). 13C NMR: d 56.4, 56.7, 60.7, 107.5, 114.9, 119.0, 121.3, 121.6, 123.6, 130.6, 140.7, 146.0, 152.2, 153.7, 156.5, 193.8. MS: m/z (%) 344 (M+, 100), 295, 194, 179, 151, 121, 107, 91, 65, 53.
2′-Hydroxy-3,4,5-trimethoxy-5′-methyl-chalcone (3i)
Red powder; mp 115oC; 1H NMR: 2.4 (s, 3H), 3.9 (s, 3H), 4.0 (s, 6H), 7.0 (m, 3H), 7.3 (d, J = 8.8 Hz, 1H), 7.5 (d, J = 15.4 Hz, 1H), 7.7 (s, 1H), 7.8 (d, J = 15.4 Hz, 1H), 12.7 (s, 1H). 13C NMR: d 20.7, 56.3, 56.4, 61.1, 103.3, 106.1, 118.5, 119.5, 119.7, 128.0, 129.3, 130.2, 137.6, 140.8, 145.6, 153.6, 161.7, 193.5. MS: m/z (%) 328 (M+, 100), 194, 181, 161, 135, 105, 77, 51.
5′-Chloro-2′-hydroxy-3,4,5-trimethoxychalcone (3j)
Yellow powder; mp 170-172oC; 1H NMR: 3.9 (s, 3H), 4.0 (s, 6H), 6.9 (s, 1H), 7.0 (d, J = 8.8 Hz, 2H), 7.4 (s, 1H), 7.5 (d, J = 15.4 Hz, 1H), 7.8 (s, 1H), 7.9 (d, J = 15.4 Hz, 1H), 12.8 (s, 1H). 13C NMR: d 56.4, 61.2, 106.2, 118.5, 120.4, 120.7, 123.5, 128.8, 129.8, 136.2, 141.2, 146.9, 153.6, 162.2, 192.7. MS: 348 (M+, 100), 194, 181, 155, 127, 83.
3′,5′-Dichloro-2′-hydroxy-3,4,5-trimethoxychalcone (3k)
Red powder; mp 144-146oC; 1H NMR: 3.9 (s, 3H), 4.0 (s, 6H), 6.9 (s, 1H), 7.3 (s, 1H), 7.4 (d, J = 15.4 Hz, 1H), 7.6 (d, J = 2.2 Hz, 1H), 7.8 (d, J = 2.2 Hz, 1H), 8.0 (d, J = 15.4 Hz, 1H), 13.5 (s, 1H). 13C NMR: d 56.4, 61.2, 106.4, 118.0, 121.2, 123.2, 124.3, 127.4, 129.6, 135.8, 147.9, 153.7, 192.4. MS: m/z (%) 384 (M++1, 69), 351, 194, 179, 151, 133, 77, 63.
5′-Bromo-2′-hydroxy-3,4,5-trimethoxychalcone (3l)
Yellow powder; mp 165-167oC; 1H NMR: 3.9 (s, 3H), 4.0 (s, 6H), 6.9-7.6 (m, 5H), 7.9 (d, J = 15.4 Hz, 1H), 8.0 (d, J= 15.4 Hz, 1H), 12.8 (s, 1H). 13C NMR: d 56.5, 61.2, 106.3, 110.5, 118.5, 120.8, 121.4, 129.8, 131.8, 139.0, 141.3, 146.9, 153.7, 162.6, 192.6. MS: m/z (%) 394 (M++1, 78), 194, 181, 168, 145, 119, 63.
2′-Hydroxy-3,4,5,4′-tetramethoxychalcone (3m)
Yellow powder; mp 118-119oC; 1H NMR: 3.8 (s, 3H), 3.9 (s, 3H), 4.0 (s, 6H), 6.5 (d, J = 2.2 Hz, 2H), 6.9 (s, 2H), 7.5 (d, J = 15.4 Hz, 1H), 7.8 (d, J = 15.4 Hz, 1H), 7.8 (s, 1H), 13.5 (s, 1H). 13C NMR: d 55.7, 56.3, 61.1, 101.1, 105.9, 107.9, 114.1, 119.6, 130.3, 131.3, 140.7, 144.6, 153.6, 166.3, 166.8, 191.7. MS: m/z (%) 344 (M+, 100), 194, 181, 151, 135, 77, 63.
2′-Hydroxy-3,4,5,6′-tetramethoxychalcone (3n)
Yellow powder; mp 123-124oC; 1H NMR: 3.8 (s, 3H), 3.9 (s, 6H), 4.0 (s, 3H), 6.5 (d, J = 8.08 Hz, 1H), 6.6 (d, J = 8.1 Hz, 1H), 6.9 (s, 2H), 7.4 (d, J= 15.5 Hz, 1H), 7.7 (s, 1H), 7.8 (d, J = 15.5 Hz, 1H,), 13.1 (s, 1H). 13C NMR: d 56.0, 56.3, 61.1, 101.7, 105.8, 111.1, 112.1, 127.1, 131.0, 136.0, 143.1, 153.5, 161.0, 164.9, 194.3. MS: m/z (%) 344 (M+, 100), 313, 194, 181, 151, 121, 91, 63.
2,4-Dichloro-2′-hydroxychalcone (3o)
Yellow powder; mp 150-152oC (lit.[7a] mp 178-180oC); 1H NMR data is in agreement with Lit[7a]. 13C NMR: d 118.8, 119.1, 119.9, 123.1, 127.7, 128.7, 129.8, 130.4, 131.6, 136.4, 136.8, 136.9, 140.0, 163.8, 193.3. MS: m/z (%) 294 (M++1, 12), 257, 165, 136, 121, 99, 65.
2,4-Dichloro-2′-hydroxy-5′-methoxychalcone (3p)
Red powder; mp 140oC; 1H NMR: 3.8 (s, 3H), 7.0 (d, J = 8.8 Hz, 1H), 7.1-7.3 (m, 3H), 7.5 (d, J = 2.2 Hz, 1H), 7.6 (d, J = 15.4 Hz, 1H), 7.7 (d, J = 8.8 Hz, 1H), 8.2 (d, J = 15.4 Hz, 1H), 12.2 (s, 1H). 13C NMR: d 56.2, 113.0, 119.5, 119.6, 123.3, 124.3, 127.7, 128.7, 130.4, 131.6, 136.4, 136.9, 137.0, 140.1, 151.8, 158.1, 193.0. MS: m/z (%) 324 (M++1, 75), 150, 135, 122, 99, 75.
2,4-Dichloro-2′-hydroxy-5′-methylchalcone (3q)
Red powder; mp 138-140oC; 1H NMR: 2.3 (s, 3H), 7.0 (d, J = 8.8 Hz, 1H), 7.2 (d, J = 15.4 Hz, 1H), 7.3 (m, 2H), 7.5 (d, J = 2.2 Hz, 1H), 7.6 (s, 1H), 7.7 (d, J = 8.04 Hz, 1H), 8.2 (d, J = 15.4 Hz, 1H), 12.5 (s, 1H). 13C NMR: d 20.7, 118.6, 119.6, 123.2, 127.7, 128.1, 128.7, 129.4, 130.3, 131.7, 136.4, 136.9, 137.9, 139.7, 161.7, 193.2. MS: m/z (%) 307 (M+, 11), 271, 161, 135, 106, 99, 77.
2,4,5′-Trichloro-2′-Hydroxy-chalcone (3r)
Yellow powder; mp 175-176oC; 1H NMR: 6.9 (d, J = 8.8 Hz, 1H), 7.3-7.4 (m, 3H), 7.5 (d, J = 15.4 Hz, 1H), 7.7 (d, J = 8.0 Hz, 1H), 7.8 (d, J = 3.0 Hz, 1H,), 8.3 (d, J = 15.4 Hz, 1H), 12.6 (s, 1H). 13C NMR: d 120.5, 122.3, 123.8, 127.7, 127.8, 128.8, 129.9, 130.4, 131.3, 136.6, 136.4, 137.4, 141.0, 162.2, 192.4. MS: m/z (%) 328 (M++1, 20), 291, 181, 155, 136, 126, 99, 75.
2,4,3′,5′-Tetrachloro-2′-Hydroxy-chalcone (3s)
Yellow powder; mp 144-146oC; 1H NMR: 7.3-7.6 (m, 5H), 7.8 (d, J = 15.4 Hz, 1H), 8.3 (d, J = 15.4 Hz, 1H), 13.2 (s, 1H). 13C NMR: d 120.9, 121.8, 123.5, 127.5, 127.8, 128.9, 130.5, 131.0, 136.2, 136.8, 137.7, 142.0, 158.1, 192.2. MS: m/z (%) 362 (M+, 9), 327, 188, 160, 135, 109, 99, 75.
5′-Bromo-2,4-dichloro-2′-hydroxychalcone (3t)
Yellow powder; mp 170-172oC; 1H NMR: 6.9 (d, J = 8.8 Hz, 1H), 7.3-7.8 (m, 5H), 7.9 (d, J = 15.4 Hz, 1H), 8.3 (d, J= 15.4 Hz, 1H), 12.6 (s, 1H). 13C NMR: d 110.6, 120.9, 122.3, 127.8, 128.8, 130.4, 131.9, 136.6, 137.4, 139.4, 141.0, 162.7, 192.3. MS: m/z (%) 372 (M+, 80), 99, 75.
2,4-Dichloro-2′-Hydroxy-4′-methoxychalcone (3u)
Yellow powder; mp 185-187oC; 1H NMR: 3.9 (s, 3H), 7.0 (m, 2H), 7.3-7.4 (m, 2H), 7.5 (d, J = 15.4 Hz, 1H), 7.7 (d, J = 8.8 Hz, 1H), 7.8 (d, J = 8.8 Hz, 1H), 8.2 (d, J = 15.4 Hz, 1H), 13.3 (s, 1H). 13C NMR: d 55.8, 101.2, 108.1, 114.0, 123.4, 127.7, 128.6, 130.3, 131.3, 131.8, 136.2, 136.7, 138.9, 166.6, 166.9, 191.3. MS: m/z (%) 323 (M+, 100), 150.10, 99, 75.
2,4-Dichloro-2′-hydroxy-6′-methoxychalcone (3v)
Red powder; mp 163-166oC; 1H NMR: 3.9 (s, 3H), 6.4 (d, J = 8.1 Hz, 1H), 6.6 (d, J = 8.1 Hz, 1H), 7.3-7.4 (m, 3H), 7.6 (d, J = 8.0 Hz, 1H), 7.8 (d, J = 15.4 Hz, 1H), 8.1 (d, J = 15.4 Hz, 1H), 13.0 (s, 1H). 13C NMR: d 56.1, 101.6, 111.1, 111.9, 127.6, 128.6, 130.2, 130.4, 132.3, 136.1, 136.2, 136.4, 137.2, 161.0, 165.1, 194.0. MS: m/z (%) 323 (M+, 100), 150, 99, 75.
6-Chloroflavanone (4a)
Pale yellow powder; mp 82-83oC; 1H and 13C NMR data is in agreement with Lit[17]. MS: m/z (%) 258 (M+, 86), 154, 126, 104, 77, 63.
6-Methoxyflavanone (4b)
Yellow powder; mp 133-135oC (lit.[14c] mp 142-143oC); 1H and 13C NMR data is in agreement with Lit[14c]. MS: m/z (%) 254 (M+, 71), 177, 150, 135, 122, 104, 77, 51.
6-Methylflavanone (4c)
Yellowish white powder; mp 99-100oC; 1H NMR: 2.3 (s, 3H), 2.9 (dd, J = 16.8, 2.9 Hz, 1H), 3.1 (dd, J = 16.8, 13.2 Hz, 1H), 5.5 (dd, J = 13.2, 2.9 Hz, 1H,), 6.9-7.7 (m, 8H). 13C NMR: d 20.5, 44.8, 79.7, 118.0, 126.2, 126.7, 128.4, 128.8, 131.4, 137.4, 139.0, 159.7, 192.3. MS: m/z (%) 238 (M+, 64), 161, 134, 105, 77, 51.
6,8-Dichloroflavanone (4d)
Yellow powder; mp 104-106oC; 1H NMR: 3.0 (dd, J = 17.0, 3.6 Hz, 1H), 3.1 (dd, J = 17.0, 13.0 Hz, 1H), 5.6 (dd, J = 13.0, 3.6 Hz, 1H), 7.5-7.8 (m, 7H). 13C NMR: d 43.9, 80.1, 122.6, 124.4, 125.2, 126.9, 129.9, 129.1, 135.8, 137.8, 155.8, 190.1. MS: m/z (%) 294 (M++1, 43), 190, 188, 160, 132, 104, 77, 51.
6-Bromoflavanone (4e)
Yellow powder; mp 103-105oC; 1H and 13C NMR data is in agreement with Lit[17]. MS: m/z (%) 303 (M+, 77), 254, 227, 198, 170, 150, 104, 77, 63.
6,4′-Dichloroflavanone (4f)
Yellowish white powder; mp 148-150oC; 1H NMR: 2.9 (dd, J = 17.0, 2.9 Hz, 1H,), 3.0 (dd, J = 17.0, 13.3 Hz, 1H), 5.5 (dd, J = 13.3, 2.9 Hz, 1H), 7.0-7.9 (m, 7H). 13C NMR: d 43.7, 77.4, 119.9, 121.8, 126.5, 127.5, 127.6, 129.3, 135.0, 135.9, 136.3, 155.5, 189.7. MS: m/z (%) 293 (M+, 17), 154, 126, 98, 75, 63.
4′-Chloro-6-Methyl-flavanone (4g)
White powder; mp 118-120oC; 1H NMR: 2.3 (s, 3H), 2.9 (dd, J = 17.0, 3.0 Hz, 1H), 3.0 (dd, J = 17.0, 13.2 Hz, 1H), 5.4 (dd, J = 13.2, 3.0 Hz, 1H,), 6.9-7.4 (m, 7H). 13C NMR: d 20.5, 44.7, 77.4, 117.9, 120.6, 126.7, 129.1, 131.4, 134.6, 137.5, 159.5, 191.9. MS: m/z (%) 272 (M+, 54), 161, 134, 105, 77, 51.
6,8,4′-Trichloroflavanone (4h)
White powder; mp 131-133oC; 1H NMR: 2.9 (dd, J = 17.0, 3.0 Hz, 1H), 3.1 (dd, , J = 17.0, 12.0 Hz, 1H), 5.6 (dd, J = 12.0, 3.0 Hz, 1H), 7.3-7.8 (m, 6H). 13C NMR: d 43.8, 77.5, 122.6, 124.4, 125.2, 127.2, 129.3, 135.0, 135.9, 136.3, 155.5, 189.7. MS: m/z (%) 328 (M++1, 33), 188, 138, 77, 51.
4′-Chloro-6-methoxyflavanone (4i)
Pale yellow powder; mp 104oC (lit.[14c] mp 114-115oC); 1H and 13C NMR data is in agreement with Lit[14c]. MS: m/z (%) 288 (M+, 32), 150, 135, 122, 103, 77, 51.
6-Bromo-4′-chloroflavanone (4j)
Yellowish powder; mp 157-159oC; 1H NMR: 2.9 (dd, J = 16.8, 2.9 Hz, 1H), 3.0 (dd, J = 16.8, 13.2 Hz, 1H), 5.4 (dd, J = 13.2, 2.9 Hz, 1H), 6.9-8.0 (m, 7H). 13C NMR: d 44.2, 77.4, 114.6, 120.3, 122.2, 127.6, 129.2, 129.6, 134.9, 136.8, 138.9, 160.2, 190.3. MS: m/z (%) 338 (M++1, 40), 198, 170, 138, 103, 77, 63.
References
- Dimmock J.R., Elias D.W., Beazely M.A., Kandpu N.M., Curr. Med. Chem., 6: 1125 (1999).
- (a) Boumendjel A., Ronot X., Boutonnat J., Curr. Drug Targets., 10: 363 (2009); (b) Katsori A.M., Hadjipavlou-Litina D., Curr. Med. Chem., 16: 1062 (2009); (c) Won S.J., Liu C.T., Tsao L.T., Weng, J.R., Ko, H.H., Wang, J.P., Lin, C.N., Eur. J. Med. Chem., 40: 103 (2005); (d) Cabrera M., Simoens M., Falchi G., Lavaggi M.L., Piro O.E., Castellano E.E., Vidal A., Azqueta A., Monge A., de Cerain A.L., Sagrera G., Seoane G., Cerecetto H., Gonzalez M., Bioorg. Med. Chem., 15: 3356 (2007); (e) Navarini-Formento A.L., Chiaradia L.D., Mascarello A., Fritzen M., Nunes R.J., Yunes R.A., Creczynski-Pasa T.B., Eur. J. Med., Chem., 44: 1630 (2009); (f) Vogel S., Heilmann J., J. Nat. Prod., 71: 1237 (2008); (g) Vogel S., Ohmayer S., Brunner G., Heilmann J., Bioorg. Med. Chem., 16: 4286 (2008).
- Ducki S., Anticancer agents Med. Chem., 9: 336 (2009).
- (a) Kontogiorgis C., Mantzanidou M., Hadjipavlou-Litina D., Mini. Rev. Med. Chem., 8: 1224 (2008); (b) Maria K., Dimitra H.L., Maria G., Med. Chem., 4: 586 (2008); (c) Arockia Babu M., Shakya N., Prathipati P., Kaskhedikar S.G., Saxena A.K. Bioorg. Med. Chem., 10: 4035 (2002); (d) Kim Y.H., Kim J., Park H., Kim H.P., Biol. Pharm. Bull., 30: 1450 (2007); (e) Capasso A., Pinto A., Mascolo N., Autore G., Capasso F., Phytother. Res., 5: 85 (1991); (f) Dao T.T., Chi Y.S., Kim J., Kim H.P., Kim S., Park H., Bioorg. Med. Chem. Lett., 14: 1165 (2004); (g) Chiaradia L.D., Dos Santos R., Vitor C.E., Vieira A.A., Leal P.C., Nunes R. J., Calixto J.B., Yunes R.A., Bioorg. Med. Chem., 16: 658 (2008); (h) Gomes A., Fernandes E., Lima J.L., Mira L., Corvo M.L., Curr. Med. Chem., 15: 1586 (2008); (i) Thanh-Dao T., Haeil P., Hyun P.K., Gerhard F.E., Khac-Minh T., Bioorg. Med. Chem. Lett., 19: 1650 (2009); (j) Nowakowska Z., Eur. J. Med. Chem., 42: 125 (2007).
- (a) Tomar V., Bhattacharjee G., Kamaluddin S., Srivastava R.K., Puri S.K., Eur. J. Med. Chem., 45: 745 (2010); (b) Renate H.H., Guantai E.M., Lategan C., Smith P.J., Wan B., Franzblau S.G., Gut J., Rosenthal P.J., Chibale K., Bioorg. Med. Chem. Lett., 20: 942 (2010); (c) Kaur K., Jain M., Kaur T., Jain R., Bioorg. Med. Chem., 17: 3229 (2009).
- Mojzis J., Varinska L., Mojzisova G., Kostova I., Mirossay L., Pharmacol. Res., 57: 259 (2008).
- (a) Prasad Y.R., Rao A.L., Rambabu R., E-J. Chem., 5: 461 (2008); (b) Nowakowska Z., Kedzia B., Schroeder G., Eur. J. Med. Chem., 43: 707 (2008); (c) Sivakumar P.M., Priya S., Doble M., Chem. Biol. Drug. Des., 73: 403 (2009); (d) Avila H.P., Smania E.F.A.F.; Monache D., Junior A.S., Bioorg. Med. Chem., 16: 9790 (2008).
- Ohkatsu Y., Satoh T., J. Jpn. Petrol. Inst., 51: 298 (2008).
- (a) Boumendjel A., Boccard J., Carrupt P.A., Nicolle E., Blanc M., Geze A., Choisnard L., Wouessidjewe D., Matera E.L., Dumontet C., J. Med. Chem., 51: 2307 (2008); (b) Pouget, C., Lauthier F., Simon A., Fagnere C., Basly J-P., Delage C., Chulia A-J., Bioorg. Med. Chem. Lett., 11: 3095 (2001).
- (a) Benavente-Garcia O., Castillo J., J. Agric. Food Chem., 56: 6185 (2008); (b) Rajkapoor B., Murugesh N., Rama K.D., Nat. Prod. Res., 23: 1384 (2009).
- (a) Hoffmann J.J., Wachter G.A., Gutteman J.U., US Patent 6136849; (b) Wang Y.,Tan W., Li W.Z., Li Y., J. Nat. Prod., 64: 196 (2001).
- Kim Y.C., Kim H-S., Wataya Y., Sohn D.H., Kang T.H., Kim M.S., Kim Y.M., Geon-Mok Lee G-M., Chang J-D., Park H., Biol. Pharm. Bull., 27: 748 (2004).
- (a) Dhar D.N., The Chemistry of Chalcones and Related Compounds; John Wiley and Sons: New York, 1981. (b) Stoyanov E.V., Champavier Y., Simon A., Basly J-P., Bioorg. Med. Chem. Lett., 12: 2685 (2002); (c) Saravanamurugan S., Palanichamy M. Arabindoo B., Murugesan V., Catal. Commun., 6: 399 (2005); (d) Ballesteros J.F., Sanz M.J., Ubeda A., Miranda M.A. Iborra S., Paya M., Alcaraz M.J., J. Med. Chem., 38: 2794 (1995); (e) Tran T-D., Park H., Kim H.P., Ecker G.F. Thai K-M., Bioorg. Med. Chem. Lett., 19: 1650 (2009); (f) Casiraghi G., Casnati G., Dradi E., Messori R., Sartori G., Tetrahedron, 35: 2061 (1979); (g) Macquarrie D.J., Nazih R., Sebti S., Green Chem., 4: 56 (2002); (h) Barros A.I.R.N.A., Silva A.M.S., Alkorta I., Elguero J., Tetrahedron, 60: 6513 (2004); (i) Detsi A., Majdalani M., Kontogiorgis C. A., Dimitra H-L., Kefalas P., Bioorg. Med. Chem., 17: 8083 (2009); (j) Climent M.J., Corma A., Iborra S., Primo J., J. Catal., 151: 60 (1995); (k) Lorenz M., Kabir M.S., Cook J.M., Tetrahedron Lett., 51: 1095 (2010).
- (a) Sagreraa G.J., Seoane G.A., J. Braz. Chem. Soc., 16: 851 (2005); (b) Choudary B.M., Ranganath K.V.S., Yadav J., Kantam M.L., Tetrahedron Lett., 46: 1369 (2005); (c) Lee J.I., Jung M.G., Bull. Kor. Chem. Soc., 28: 859 (2007); (d) Dauzonne D., Monneret C., Synthesis 11: 1305 (1997); (e) Wang X., Cheng S., Catal. Commun., 7: 689 (2006); (f) Chaturvedi R., Patil P.N. Mulchandani N.B., Indian J. Chem., 31B: 340 (1992); (g) Wang L., Liu X., Dong Z.,Fu X.,Feng X., Angew. Chemie Int. Ed., 47: 8670 (2008).
- Alkhathlan H.Z., Synth. Commun., 34: 71 (2004).
- (a) Al-Bogami A.S., Al-Majid A.M., Al-Saad M.A., Mousa A.A., Al-Mazroa S.A., Alkhathlan,H.Z., Molecules, 14: 2147 (2009); (b) Alkhathlan H.Z., Tetrahedron, 59: 8163 (2003); (c) Alkhathlan H.Z., Al-Saad M.A. Al-Hazmi H.M., Al-Farhan K.A., Mousa A.A., J. Chem. Res., (M) 1023: (S) 473 (2002); (d) Alkhathlan H.Z., Al-Saad, M.A., Al-Hazmi H. M., Al-Farhan K.A., Mousa A.A., J. Chem. Res., (M) 1201: (S) 587 (2002); (e) Alkhathlan H.Z., Al-Farhan K.A., Heterocycles, 48: 641 (1998); (f) Alkhathlan H.Z., Heterocycles, 45: 45 (1997); (g) Alkhathlan H.Z., J. Chem. Res., (M) 1984: (S) 260 (1992).
- Bovicelli P., Bernini R., Antonioletti R., Tetrahedron Lett., 43: 5563 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









