Qualitative and Quantitative Analysis of Metal Complexes of Schiff Bases Containing Medicinally Important Amino Compounds
Amit Kumar Saxena and Rajnish Saxena
Department of Chemistry, S.M.College, Chandausi - 202 412 (India).
Mixed ligand octahedral Titanium(III) complexes with the tridentate salicylaldehydesemi-, thiosemi- and isothiosemicarbazone and pyridine of general formula [TiIII(L1-3)(py)3]X(H2L1 = salicylaldehyde semicarbazone,X=[TiIICl3(py)]-, ClO4- .H2O, I- . 0.5 I2; H2L2 = salicylaldehyde thiosemicarbazone, X = [TiIICl3(py)]-,[TiIIBr3(py)]-, ClO4- . H2O, I3-; H2L3 = salicylaldehyde S-methylisothiosemicarbazone,X = [TiIIBr3(py)]-, ClO4- . H2O, BF4-) were synthesized. The tridentate coordination of all the three dianionic forms of the ligands involves the phenol oxygen,hydrazine nitrogen and the chalcogen (O or S) in case of salicylaldehyde semi-,thiosemicarbazone and the terminal nitrogen atom in the case of isothiosemicarbazone.For all the complexes, a meridial octahedral arrangement is proposed, which is a consequence of the planarity of the chelate ligand. The compounds were characterized by elemental analysis, molar conductivity, magnetic susceptibility, IR and electronic absorptionspectra. The thermal decomposition of the complexes was investigated by thermogravimetry, coupled TG-MS measurements and DSC.
KEYWORDS:mixed Ti(III) complexes; salicylaldehyde semi-; thiosemi-; isothiosemicarbazone; pyridine; Titanium Complexes with Semicarbazone and Thiosemicarbazone
Download this article as:| Copy the following to cite this article: Saxena A. K, Saxena R. Qualitative and Quantitative Analysis of Metal Complexes of Schiff Bases Containing Medicinally Important Amino Compounds. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Saxena A. K, Saxena R. Qualitative and Quantitative Analysis of Metal Complexes of Schiff Bases Containing Medicinally Important Amino Compounds. Available from: http://www.orientjchem.org/?p=23463 |
Introduction
Due to their good complexing properties, biological activity and analytical application, semi-/thiosemi-/isothiosemicarbazides and their Schiff bases of different denticity, as well as their metal complexes, have been subject of many studies. Apparently, the most numerous among them are the complexes with tridentate salicylaldehyde semi-/thiosemi-/isothiosemicarbazones. In contrast to salicylaldehyde semi-/thiosemicarbazones, whose donor atoms are O, N, X (X=O or S), the third donor atom with isothiosemicarbazone derivatives is the nitrogen of the isothioamide group.
Titanium(III) and various tridentate ligands form mainly mixed bis(ligand) complexes,whereas mixed complexes such as [Ti(L)A3]0,[Ti(L)A2B]+,[Ti(L)B3]3+
(L=diethylenetriamine, cyclohexanitroamine, bis(2-aminoethyl)-sulphide,N-(3-aminopropyl)-1,3-propantriamine; A=NO2, N3, CN, Cl; B=NH3), are much rarer.in this study, the syntheses and some physico-chemical characteristics of Ti(III) complexes with salicylaldehyde semi- (H2L1), thiosemi- (H2L2) and S-methylisothiosemicarbazone (H2L3) are presented.
Experimental
Reagents: All chemicals used were commercially available products of analytical reagent grade, except for the ligands salicylaldehyde semicarbazone (H2L1), thiosemicarbazone (H2L2) and S-methylisothiosemicarbazone (H2L3).
Synthesis of the complexes: [Ti(L1)(py)3][TiCl3(py)]. EtOH (5.0 cm3 ) and pyridine (~5 mmol) were added to a mixture of TiCl2.6H2O(1.2 mmol) and salicylaldehyde semicarbazone, (H2L1) (0.6 mmol) in the presence of LiOAc (4mmol). The reactants were dissolved by stirring and mild heating. After 4 days at room temperature, the green crystals were separated by filtration and washed with EtOH and Et2O. The yield was 0.27 g (61 %).
[Ti(L2)(py)3][TiCl3(py)] and [Ti(L2,3)(py)3][TiBr3(py) ]. EtOH (5.0 cm3) and pyridine (~5 mmol) were added to a mixture of TiX2.6H2O (X = Cl, Br) (2.5 mmol) and salicyladehyde thiosemi-(H2L2)/S-methylisothiosemicarbazone (H2L3) (1.25 mmol). After 24 h, the green crystals were separated by filtration and washed with EtOH and Et2O. The yield was 0.10 g (11 %), 0.29 g (27 %), 0.48 g (52 %) respectively.
[Ti(L1-3)(py)3]ClO4.H2O. To a mixture of the ligands (H2L1–3) (0.5 mmol) and Ti(ClO4)2 (1 mmol),EtOH (5.0 cm3) and pyridine (~5 mmol) were added, and the mixture was heated for a fewminutes. After 24h, the brown crystals were separated by filtration and washed with EtOH and Et2O. The yield was 0.27 g (63%), 0.20 g (64 %), 0.19 g (65 %), respectively.
[Ti(L1)(py)3]I.0.5I2 and [Ti(L2)(py)3]I3. To a warm solution of NaI (5 mmol) in EtOH (5.0 cm3), 2.5 mmol TiCl2.6H2O were added and the resulting solution was heated for a few minutes. After 15 min the precipitated NaCl was separated by filtration. To the TiI2 solution was then added H2L1/H2L2 (1.25 mmol) and pyridine (~5mmol), and themixture was dissolved by heating. After 24 h, the obtained brown crystals were separated by filtration and washed with EtOH and Et2O. The yield was 0.30 g (34 %), 0.30 g (28 %), respectively.
Structural formulas of salicylaldehyde semi- (H2L1), thiosemi- (H2L2) andS-methylisothiosemicarbazone (H2L3).[Ti(L3)(py)3]BF4. Amixture of TiCl2.6H2O (2 mmol) and NaBF4 (4 mmol) in EtOH (6.0 cm3) was heated for a few minutes.After 15 minutes, the precipitatedNaClwas separated by filtration. To theTi(BF4)2 solutionwere then addedH2L3 (2 mmol) and pyridine (5mmol), and themixture was dissolved by heating.After 2 days, the obtained greenmonocrystals of composition Ti(L3)(py)3TiCl3(py)·EtOH11 were separated by filtration. After 24 h (r. t.), the brown crystals which formed in the filtratewere separated by filtration and washed with EtOH and Et2O. The yield was: 0.33 g (28 %).
Analytical Methods
Elemental analysis (C, H, N) was carried out by standard micromethods.
The content of the metal in the complexes was determined after previous sample decomposition by heating in a Kjeldahl flask in conc.H2SO4 and conc.HNO3, followed by evaporation to dryness. The dry residue was dissolved in water and the metal content determined by complexometric titration (EDTA).
Magnetic susceptibility measurements were performed at room temperature using an MSB-MKI magnetic susceptibility balance. The data were corrected for diamagnetic susceptibilities.
Molar conductivities of freshly prepared 1×10-3 mol/dm3 DMF solutions were measured using a Janway 4010 conductivity meter.
IR spectra (KBr disc) were recorded using a Perkin-Elmer 457 Infracord spectrophotometer.
Electronic absorption spectra were recorded using a Carl Zeiss spectrophotometer.
Thermalmeasurements were carried out in dynamic air and argon atmospheres at a heating rate of 10K min-1. The thermogravimetric curves were registered up to 1000 K by means of a DuPont 2000 TAsystem with a thermobalanceDuPont 951 TGAusing sample masses of about 5mg in a platinum crucible. The DSC curves were recorded up to 600 K in an open aluminium pan as the sample holder with an empty aluminium pan as the reference. TG-MS measurements were performed on a TA Instruments SDT 2960 coupled with Balzers Thermostar GSD 300 T capillary MS in dynamic helium and air atmospheres.
Results and Discussion
Mixed-ligand octahedral complex of cobalt(III) with salicylaldehyde semi- (H2L1),
thiosemi- (H2L2) and S-methylisothiosemicarbazone (H2L3), of the type TiIII(L)(py)3X(L = L1, X = [TiIICl3(py)]–, ClO4–.H2O, I–.0.5I2; L = L2, X = [TiIICl3(py)]–,[TiIIBr3(py)]–, ClO4–.H2O, I3–; L = L3, X = [TiIIBr3(py)]–, ClO4–.H2O, BF4–) were obtained by reacting warm ethanolic solutions of Titanium(II) salts and the mentioned ligands and pyridine in the ratio 2:1:5. In the first stage of the preparation of the complexwith BF4–, the complex[Ti(L3)(py)3][TiCl3(py)].EtOH11 was formed, which means that the metathetical reaction between TiCl2 and NaBF4 yielded no complete precipitation of the chloride (NaCl). It should bementioned that attempts to isolate complexes with the mixed anion [TiIII3(py)]– were unsuccessful.
All the complexes are well soluble in DMF, less in MeOH, EtOH andMe2CO,and insoluble in H2O and Et2O.
On the basis of the obtained results it can be concluded that the complexes [TiIII(L1–3)(py)3]+ are formed only in combination with large counterions,such as [TiIIX3(py) ]– (X=Cl,Br) or ClO4–. The formation of complexes with the smaller BF4– ion is hindered, which was observed in the synthesis of Ti(L3)(py)3]BF4, as this complex could be obtained only after separation of the primarily formed[TiIII(L3)(py)3][TiIICl3(py)].
The necessity of the presence of a relatively large anion in combination with the large [TiIII(L1–3)(py)3]+ cation has also been confirmed on the examples of the[TiIII(L2)(py)3]I3 complex, similar to the previously synthesized isothiosemicarbazone complexes,11 such as [TiIII(L1)(py)3]I.0.5I2, in which a significant interaction exists between I2 and two I– anions from the neighbouring molecules. A similar situation was also found in the crystal structure of the [TiIII(H2L)I2]I.0.5I2(H2L = bis(hydrazone 2,6 diacetylpyridine)) complex.
Table 1: Some physico-chemical characteristics and analytical data of the complexes
| Complex | Colour | µeff/µB | λM (Scm2/mol) | Found (Calcd.) / % | |||
| C | H | N | Ti | ||||
| [Ti(L1)(py)3][TiCl3(py)] | Green | 4.38 | 43.5 | 47.34(46.85) | 4.07(3.79) | 13.95(13.66) | 15.94(16.42) |
| [Ti(L2)(py)3][TiCl3(py)] | Green | 4.42 | 44.6 | 46.14(45.83) | 4.03(3.71) | 13.10(13.66) | 16.27(16.06) |
| [Ti(L2)(py)3][TiBr3(py)] | Green | 4.22 | 101.6 | 38.08(38.77) | 3.61(3.14) | 11.14(11.30) | 13.27(13.59) |
| [Ti(L3)(py)3][TiBr3(py)] | Green | 4.96 | 157.4 | 38.89(39.52) | 3.66(3.32) | 11.04(11.12) | 13.03(13.37) |
| [Ti(L1)(py)3]ClO4.H2O | Brown | Diam | 60.9 | 47.22(46.75) | 4.25(4.10) | 14.05(14.22) | 9.42(9.97) |
| [Ti(L2)(py)3]ClO4.H2O | Brown | Diam | 67.7 | 46.06(45.51) | 4.10(3.99) | 13.35(13.58) | 9.41(9.69) |
| [Ti(L3)(py)3]ClO4.H2O | Brown | Diam | 82.8 | 44.50(46.42) | 3.35(4.22) | 13.54(13.58) | 9.15(9.49) |
| [Ti(L1)(py)3]I0.5I2 | Brown | Diam | 68.5 | 41.94(37.99) | 3.78(3.05) | 12.33(11.55) | 8.00(8.10) |
| [Ti(L1)(py)3]I3 | Brown | Diam | 23.4 | 33.45(31.75) | 2.79(2.55) | 11.08(9.66) | 6.46(6.67) |
| [Ti(L1)(py)3]BF4 | Brown | Diam | 54.2 | 49.07(48.83) | 4.57(4.10) | 13.20(14.24) | 10.21(9.98) |
Finally, the obtained results indicate the differences in the possibility of the formation of tri-halogenopyridinetitate(II) ions which, to our knowledge, have only been found in combination with the complex cation [TiIII(L1–3)(py)3]+.Namely, under identical experimental conditions, the [TiCl3(py)]– anion is very easily formed, [TiBr3(py)]– is much more difficult to prepare, whereas the analogous iodo-complex is not formed at all.
As can be seen from their coordination formulas (Table I), all the complexes contain the same complex cation [TiIII(L1–3)(py)3]+ with dianionic form of the Schiff bases formed by deprotonation of the most acidic phenolic OH group and deprotonated enolised keto/thioketo group in the case of H2L1,2, and the isothioamide group in the case of H2L3.
![Figure 1: Structure of [Ti(L)(py)3]+](http://www.orientjchem.org/wp-content/uploads/2012/06/vol28_iss2_ami_com_fig1-150x141.jpg) |
Figure 1: Structure of [Ti(L)(py)3]+
|
The formation of double-deprotonated form of these ligands is undoubtedly facilitated by the presence of excess pyridine. At the same time, the deprotonation of these groups is chemical proof of the participation of their donor atoms in their coordination.There is no doubt that in addition to the mentioned atoms, another participant in the coordination is the azomethine nitrogen. Thus, one six-membered (salicylidene) and one five-membered (semi-/thiosemi-/isothiosemicarbazide) metallocycles are formed, which has been confirmed by X-ray analysis of [Ti(L3)(py)3]X (X=[TiCl3 (py)]–.EtOH, I3–).Therefore, in these complexes too, thementioned ligands are coordinated in the usual tridentate mode with a meridial arrangement of the O, N, X (X = O, S, N) donor atoms.
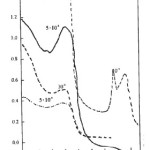 |
Figure 2: Electronic spectra of the complexes: (—) [TiIII(L2)(py)3][TiCl3(py)] in DMF; (—) [TiIII(L2)(py)3][TiCl3(py)] in Me2CO; (-.-.-)[TiIII(L2)(py)3]ClO4.H2O in DMF.
|
In concordance with the mentioned coordination mode is also the absence of the characteristic v(OH) bands in the IR spectra of the complexes, which in the ligand spectra appear at ~3470 cm–1, as well as the shift to lower energies (ca. 60cm–1) of the bands v(C=O) and v(C=S), which in the spectra of H2L1 and H2L2 appear at 1690 and 1275 cm–1 respectively. In the spectra of complexeswith H2L3, the bands v(NH) and v(NH2), observed in the ligand spectrum at 3330 and 1640cm–1, are also shifted to lower energies: 3200, 1605 cm–1, respectively. In the spectra of the complexes containing ClO4 and BF4 there are characteristic very strong single bands at ~1100 cm–1, indicating the ionic character of the acid residues.
With the exception of the complexes that contain tetrahedral anions [TiIIX3(py)]–(X = Cl, Br), all the others are diamagnetic. Magnetic moments of the paramagnetic complexes correspond to the usual values observed for tetrahedral Ti(II) complexes.
The electronic spectra of the brown DMF and Me2CO solutions of complexes are similar to each other and exhibit an absorption maximum at ~400 nm corresponding to d-d transitions of Ti(III).The green solutions of the complexes containing the tetrahedral [TiX3(py) ]– (X = Cl, Br) anion also exhibit a weak absorption in the range of 600–700 nm, belonging to d-d transitions of Ti(II).
 |
Figure 3: Thermal curves of:(—) [TiIII(L1)(py)3[[TiCl3(py)3];(—)[TiIII(L1)(py)3]I.0.5I2;(….)[TiIII(L2)(py)3][TiCl3(py)3].
|
It should be mentioned that the DMF solutions of the complexes with a tetrahedral Ti(II)-anion are evidently less stable than theMe2CO solutions of the same compounds. Namely, in DMF solutions of these complexes, known complexes of a nonelectrolyte type [TiIII(HL)(L)] are formed in the course of time, which is accompanied by a change in the colour of the solution from green to brown and by the disappearance of the absorption in the range of 600–700 nm. Such instability is most pronounced with the [TiIIBr3(py)]– ion, which is also evident from its enhanced molar conductivity in comparison with 1:1 type electrolytes. On the other hand, the molar conductivities of the other complexes are in agreement with their coordination formulas.
The thermal decomposition of all the compounds is continuous. As examples, the TG and DTG curves of selected compounds are presented in Fig. 3. The decomposition pattern does not depend on the gas carrier up to 600 K. In argon, above this temperature the decomposition rate decreases, and the decomposition is not completed up to 1000 K. In air, the decomposition of some compounds is accompanied by burning of the sample. In all cases, the decomposition of the compounds begins
with the departure of the pyridine ligand, followed by decomposition of the Schiff base and the end product isTitanium(III) oxide.
The thermal stability of the compounds is about the same and the decomposition
begins around 420 K. The highest thermal stability is exhibited by the
[Ti(L1)(py)3][TiCl3(py)] complex, which decomposes above 470 K.
In order to propose a decomposition mechanism, the decomposition of
[Ti(L)(py)3]I.0.5I2 was followed by coupled mass spectrometry up to 600 K. As the first departing group, pyridine and its decomposition products were identified. The decrease in mass supports this proposition not only in the case of the investigated compound but in the cases of other complexes too.
The DSC curves in an inert atmosphere (argon) refer to endothermic decomposition processes.
References
- G.L.Chaudhary, S.R. Prasad and A Rahman, J.Indian Chem.Soc., 683-685(1997).
- Rai P.K. & Prasad R.N., Synthreact inorg,metorg.Chem.,24:749 (1994)
- J.P.Scovill,D.L. Klaywan and C.F. Franchino, J.Med.Chem.,25:1261 (1985)
- N.K. Singh and S.K.Kushwaha, Indian Journal Of Chemistry., 333-36 (2004)
- Bost R.W.& Shealy O.L. J A M Chem Soc.25:73 (1951)
- Singh N.K., Singh S.B. Srivastava A & Singh N. Biometals 16:71 (2003)
- Singh N.K. Sharma U & Agrawal S.Transmet Chem,13:233 (1988)
- Motevalli M, Brien P.O., waish J.R. & waston I.M, Polyhedron, 15:2801 (1996)
- Singh N.K., Prasad G.C., Sodhi A & Shrivastava A, Bioorg Med Chem, 5:245 (1997)
- Argazzi R, Bignozzi C.A., Hasselman G.M. & Meyer G.J. inorg Chem 37:4533 (1998)
- Curtius T & Melsbash H, J prakt Chem, 81:501 (1910)
- Jensen K A & Pedersen C, Acta Chem Scand, 15:1087 (1961)
- Burns G R, Inorg Chem, 7:277 (1968).
- Islam M. S. & Alam M.A., J Indian Chem.Soc., 76 (1999)
- Baur A.W., Kirby W. M. Sherries J C and Turk M.Am J. Chem., Pathol, 45:493 (1996)
- Kawalendu Dey and Kartick Chakarborty.,Indian Journal of Chemistry 38A:381-384 (1999)

This work is licensed under a Creative Commons Attribution 4.0 International License.









