Preparation, Identification of two New Cd(Ii) Complexes Including 4-(1h-Tetrazol-5-Yloxy) – Phenylamine Cadmium(Ii) Chloride and 2-(1h-Tetrazol) – Pyridine Cadmium(Ii) Chloride
Shahriare Ghammamy
Departments of Chemistry, Faculty of Science, Islamic Azad University, Malard Branch, Malard (Iran).
Two Cd complexes have been prepared by reaction of Cd (II) with 4-(1H-Tetrazol-5-yloxy) – phenylamine (HTY) and 2-(1H-Tetrazol) - pyridine (HTP) ligands. These new complexes were characterized by IR, UV-Visible, 1H-NMR and 13C-NMR spectroscopies. The changes observed between the FT-IR, H-NMR and UV-Vis spectra of the ligands and complexes allowed to establish the coordination mode of the metal in complexes.
KEYWORDS:Synthesis; characterization; Cadmium Complexes; HTY; HTP
Download this article as:| Copy the following to cite this article: Ghammamy S. Preparation, Identification of two New Cd(Ii) Complexes Including 4-(1h-Tetrazol-5-Yloxy) – Phenylamine Cadmium(Ii) Chloride and 2-(1h-Tetrazol) - Pyridine Cadmium(Ii) Chloride. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Ghammamy S. Preparation, Identification of two New Cd(Ii) Complexes Including 4-(1h-Tetrazol-5-Yloxy) – Phenylamine Cadmium(Ii) Chloride and 2-(1h-Tetrazol) - Pyridine Cadmium(Ii) Chloride. Available from: http://www.orientjchem.org/?p=23441 |
Introduction
Schiff bases play an important role in inorganic chemistry as they easily form stable complexes with most transition metal ions. The development of the field of bioinorganic chemistry has increased the interest in Schiff base complexes, since it has been recognized that many of these complexes may serve as models for biologically important species [1-3].
Schiff base metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer and herbicidal applications. Oxazole is a heterocyclic organic compound that has a five-member ring molecular structure containing three carbon atoms, one oxygen atom, and one nitrogen atom [4]. It is a clear to yellowish liquid with a pyridine like odor. It is soluble in alcohol and ether and slightly soluble in water. Oxazole and its derivatives are used as building block for biochemicals and pharmaceutical as well as in other industrial applications such as pesticides, dyes, fluorescent brightening agents, textile auxiliaries and plastics. The term of isoxazole is for 1,2-oxazole [5]. These are azoles with oxygen and nitrogen separated by one carbon [6]. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole [7]. Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen and one carbon atom (plus hydrogens) [8]. The simplest is tetrazole itself, CN4H2 [9, 10]. They are unknown in nature. There are several pharmaceutical agents which are tetrazoles, but they are generally undesirable due to safety concerns for process-scale synthesis; tetrazoles are usually explosive. However, tetrazoles can act as a bioisostere for the carboxylate group, increasing their utility [11, 12].
They serve as models for biologically important species and find applications in biomimetic catalytic reactions. In this work, we report the synthesis and structural studies of the complexes isolated from the reactions of Cd (II) with two new ligands.
Material and Methods
All reagents were supplied by Merck and were used without further purification. Melting points were determined in an electrothermal 9200. The FT-IR spectra were recorded in the range 400–4000 cm-1 by KBr disk using a Brucker Tensor 27 M 420 FT-IR spectrophotometer. 13C-NMR and 1H-NMR spectra in CDCl3 were recorded on Bruker AMX-500 spectrometers The UV–Vis spectra in CH3CN were recorded with a Wpa bio Wave S2 100 spectrophotometer.
Synthesis of complex [Cd(C7H7N5O)]Cl2 (1)
Cadmium (II) chloride (0.12g, 0.65mmol) was dissolved in acetonitrile (20 ml). To this, (0.37g, 2.09mmol) HTY in ethyl acetate (20 ml) was added. The mixture was stirred magnetically. The precipitated solid was filtered, washed with hexane and dried. M.P.: 160-162 ºС. Anal. Calc. for [Cd(C7H7N5O)]Cl2 is including IR: (KBr disc): 803 (m) cm-1 υ(C-H) ,1486 (m) cm-1 υ(C-C), 1442 (s) cm-1 υ(C-N), 1627 (m) cm-1 υ(C=N), 1287 (w) cm-1 υ(C-O), 1167 (m) cm-1 υ(N-N), 3374 (w) cm-1 υ(N-H), 505 (m) cm-1 υ(Cd-N); UV–Vis (CH3CN): λmax (log ε )= 250 (225) (Figure 1, 2).
 |
Figure 1: IR spectra of (HTY) and (HTP) (top to bottom, frequencies in cm-1). |
Synthesis of Complex [Cd(C6H5N5)]Cl2 (2)
Cadmium (II) chloride (0.14g, 0.76mmol) was dissolved in water (20 ml). To this, (0.36g, 2.38mmol) HTP in ethyl acetate (20 ml) was added. The mixture was stirred magnetically. The precipitated solid was filtered, washed with hexane and dried. M.P.: 167-169 ºС. Anal. Calc.(found) for [Cd(C6H5N5)]Cl2 is including IR: (KBr disc): 721 (s) cm-1 υ(C-H), 1487 (m) cm-1 υ(C-C), 1405 (m) cm-1 υ(C-N), 1582 (w) cm-1 υ(C=N), 1161 (w) cm-1 υ(N-N), 495 (w) cm-1 υ(Cd-N); UV–Vis (CH3CN): λmax (log ε )= 275 (21) (Figure 1, 2).
Result and Discussion
Schiff bases are potentially capable of forming stable complexes with metal ions. Cadmium containing ligands are known to form stable complexes with class b metal ions Cd (II) salt react with Schiff base ligands in 1:1(L/M) molar ratio in solvent to afford complexes. The ligands and complexes are stable at room temperature. In this paper, a direct, simple and one step method has been used to synthesize these compounds. The advantages of the method are; that there is no side product, the reaction is quite fast, there are mild conditions, and the accompanied color change that provides visual means for ascertaining the progress of the reaction. In summary, the synthesis and characterization of complexes have been described. Two complexes of Cd (II) were synthesized simply. [Cd(C7H7N5O)]Cl2 and [Cd(C6H5N5)]Cl2 were prepared by the reaction of (HTY and Cd Cl2.2H2O) and (HTP and CdCl2.2H2O. All of the infrared (IR) spectra information supports the suggestion of coordination of the nitrogen and oxygen atoms to the metal ions. In this study we have reported the synthesis of two new Tetrazole ligands and theirCd (II) complexes. The structural characterizations of synthesized compounds were made by using the elemental analysis, IR and UV spectral techniques. From the spectroscopic characterization, it is concluded that ligands act as a neutral bidentate through the nitrogen atom. These [Cd(C7H7N5O)]Cl2 and [Cd(C6H5N5)]Cl2 hydrazide compounds were obtained in relatively high yield, 85 and 91% respectively. The infrared spectrum of the complex was compared with spectrum of the free ligand. This is a significant change between the metal (II) complex and free ligand for chelation as expected. The strong bands at 1627 and 1582 cm-1 are assigned to the C=N in HTY and HTP. All of the IR spectra information supports the suggestion of coordination of the nitrogen atoms to the metal ion. The complexes are stable in air and light, and are soluble in organic solvents such as DMSO and insoluble in ethanol and n-hexane. The infrared spectra of the complexes taken in the region 400–4000 cm-1 were compared with this of the free ligand. As seen data the IR spectra of metal (II) complexes are very similar to each other, except some slight shifts and intensity change of a few vibration bands caused by different metal (II) ions, which indicate that the complexes have similar structures. There are some significant changes between the metal (II) complexes and their free ligand for chelation as expected. An exhaustive comparison of the IR spectra of the complexes gave information about the mode of bonding of the ligand in metal complexes (Table 1).
Table 1: Experimental frequencies and theoretical frequencies (in cm-1) calculated by B3LYP/6- 311G* method for (HTY) and (HTP)
|
Expt
|
B3LYP/6-311G*(d,p)
|
|
|
803, 1486, 1442, 1627, 1287, 1167, 3374, 505 |
882, 1474, 1273, 1572, 1627, 1165, 3317, 530 |
(HTY) |
|
721, 1487, 1405, 1582, 1161, 495 |
882, 1474, 1395, 1572, 1165, 530 |
(HTP) |
In electronic spectra, the formations of the metal (II) complexes were also confirmed by UV–Vis spectra. The UV- Vis solution spectra of the ligands and complexes were also recorded (Figure 2).
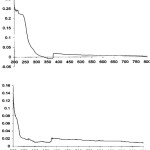 |
Figure 2: UV/Vis spectra of (HTY) and (HTP) (top to bottom, in acetonitrile, C=5×10-3M). |
After that, they were characterized by 13C-NMR and 1H-NMR For [Cd(C7H7N5O)]Cl2 13C-NMR (135 MHz, CDCl3): δ= 135.86 ppm and δ= 156.39 ppm. 1H-NMR (135 MHz, CDCl3) T= 1.85 ppm, F= 3.19 ppm and For [Cd(C6H5N5)]Cl2 13C-NMR (135 MHz, CDCl3): δ= 149.59 ppm and δ= 173.66 ppm. 1H-NMR (135 MHz, CDCl3) T= 7.54 ppm, M= 9.01 ppm.
The synthetic reactions for these complexes can be written as:
C7H7N5O + CdCl2.2H2O → [Cd(C7H7N5O)]Cl2 (1)
C6H5N5 + CdCl2.2H2O → [Cd(C6H5N5)]Cl2 (2)
CONCLUSIONS
In this study we have reported the preparation of two new Cd (II) complexes with HTY and HTP. The structural characterizations of synthesized compounds were made by using 13C-NMR, 1H-NMR IR and UV spectral techniques.
 |
Figure 3: 13C-NMR spectra of (HTY) and (HTP) (top to bottom, in CDCl3 solvent) |
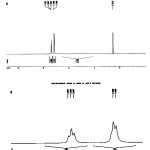 |
Figure 4: 1H-NMR spectra of (HTY) and (HTP) (top to bottom, in CDCl3 solvent) |
References
- Savanini L., Chiasserini L., Gaeta A. and Pellerano C. Bioorg. Med. Chem., 10, 2193(2002).
- Anten J. A., Nicholis D., Markpoulos J. M. and Markopoulou O. Polyhedron, 6, 1074(1987).
- Maiti A. and Ghosh S. Indian J. Chem., 28A, 980(1989).
- Jeeworth T., Wah H. L. K., Bhowon M. G., Ghoorhoo D. and Babooram K. Synt. React. Inorg. Met-Org. Chem., 30, 1023(2000).
- Dharmaraj N., Viswanalhamurthi P. and Natarajan K. Trans. Met. Chem., 26, 105(2001).
- Colins C. H. and Lyne P. M., Microhiul Methods, University Park Press, Baltimore, 422, 1970.
- Aggarwal R. C., Singh N. K. and Singh R. P. Inorg. Chim. Acta., 29, 2794(1981).
- Craliz J. C., Rub J. C., Willis D., Edger J. Nature., 34 ,176(1955).
- Cozzi P. G. Chem. Soc. Rev., 33, 410(2004).
- Tossadis I. A., Bolos C. A., Aslanidis P. N. and Katsoulos G. A. Inorg. Chim. Acta, 133, 275 1987.
- Chohan Z. H. and Sheazi S. K. A. Synth. React. Inorg. Met-Org. Chem., 29, 105(1999).
- Jayabalakrishnan C. and Natarajan K. Synt. React. Inorg. Met-Org. Chem., 31, 983(2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









