Optimization Effective Parameters on Gamma Radiation Polymerization Copolymer Based on Collagen
Mohammad Sadeghi1*, Fatemeh Shafiei2 and Esmat Mohammadinasab3
Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak (Iran).
In this work, after synthesis of graft copolymer collagen-based by gamma radiation, The effective parameters, for example, the Doses of initiator, monomer and protein as well as the reaction time on the graft copolymerization reactions were investigated to achieve the optimum grafting conditions. According to the empirical rates of the polymerization and the graft copolymerization of AMPS onto collagen backbone, the overall activation energy of the graft copolymerization reaction was estimated to be 29.8 kJ/mol.
KEYWORDS:Graft Copolymer; Collagen; 2-acrylamido-2-methylpropanesulfonic acid; Gamma Radiation
Download this article as:| Copy the following to cite this article: Sadeghi M, Shafiei F, Mohammadinasab E. Optimization Effective Parameters on Gamma Radiation Polymerization Copolymer Based on Collagen. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Sadeghi M, Shafiei F, Mohammadinasab E. Optimization Effective Parameters on Gamma Radiation Polymerization Copolymer Based on Collagen. Available from: http://www.orientjchem.org/?p=23433 |
Introduction
Graft copolymerization of hydrophilic vinyl monomers is a well-known technique employed by polymer chemists for significantly modifying the chemical and physical properties of the synthetic or natural starting materials with minimum degradation of the original properties[1-5]. Graft copolymers are prepared by first generating free radicals on the polysaccharide backbone and then allowing these radicals to serve as macroinitiators for the vinyl monomers. These biodegradable and low cost graft copolymers, with new properties can be used in many applications such as textiles, paper industry, agriculture, medical treatment, in petroleum industry as flocculants and thickening agents[2-3] and also development of selective permeable membranes[6], sorption agents[7] and in fabrication of drug delivery systems[8-9].
Proteins are widely distributed in nature and are synthesized mainly in animals, i.e. collagen, keratin, gelatin, and etc., and in a few plants such as Soya. In general, proteins are high molecular weight polymers and their solubility in aqueous solutions is difficult. Two efficient methods for preparation of aqueous soluble proteins are alkaline and enzymatic hydrolysis. According to the literature survey based on Chemical Abstract Service, a few studies have been reported in the case of graft copolymer based on protein[10-12]
Though much work has been reported on the grafting of 2-acrylamido-2-methylpropanesulfonic acid onto various polysaccharides. Therefore, The present investigation deals with the detailed study of some major factors which affect graft copolymerization of AMPS onto collagen, initiated by gamma radiation with a view to elucidate the grafting mechanism.
Materials And Methods
Material
Hydrolyzed collagen (Parvar Novin-E Tehran Co.) was industrial grade which is available in market and has nearly 25% insoluble phosphate salt. 2-acrylamido-2-methylpropanesulfonic acid (Merck, Darmstadt, Germany), were of analytical grade and used without further purification.
Graft Copolymerization Procedure
A pre-weighed amount of hydrolyzed collagen (0.5-2.5 g) was dissolved in 50 mL degassed distilled water and filtered to remove its insoluble salt. The graft copolymers were prepared by mixing monomer with bidistilled water in a test tube and finally subjected to Co-60 gamma source at 35 kGy. The dose rate was kept constant at 2.50 Gy/sec. The irradiation has been carried out using the gamma chamber cell facility in The Nuclear Radiation Center at the National Center for Radiation Research and Technology, Arak City, Iran. The The graft copolymers obtained after radiation were dried in under vacuum oven at 60°C till constant weight. An inert gas (argon) was gently bubbled into the reactor to remove the oxygen during the graft copolymerization reaction. The product was then worked up with methanol (200 mL) and dried in oven at 50 oC for 5 h.
Homopolymer extraction
The graft copolymer, collagen-g-poly(AMPS), was freed from poly (AMPS) homopolymer, by pouring 0.30 g of the product in 50 mL of dimethyl formamide solution. The mixture was stirred gently at room temperature for 48 h. After complete removal of the homopolymer by filtration of the collagen-g-poly(AMPS), copolymer, the product was washed with methanol and dried in oven at 50 oC to reach a constant weight[13].
Grafting parameters
The grafting parameters, i.e. grafting rati (Gr%), add-on value (Ad%), and homopolymer content (Hp%), used to characterize the nature of the copolymer are defined and calculated using the following equations[14-17]:
Gr % = 100 (W2 – W0) / W0 (1)
Ad %= 100 (W2 – W0) / W2 (2)
Hp % = 100 (W1 – W2) / W1 (3)
where W0, W1, and W2 are the weight of the initial substrate, total product (copolymer and homopolymer), and pure graft copolymer (after DMF extraction), respectively.
Results and Discussion
Optimization of Copolymerization Reaction
Since polymerization variables determine the extent of grafting and homopolymer amount, certain factors affecting the grafting parameters were investigated to achieve the optimum condition of polymerization. Therefore, we optimized the grafting of 2-acrylamido-2-methyl propan sulfonic acid onto Collagen in homogenous aqueous media by changing temperature, the initial concentration of monomer, initiator, and the relative amount of the substrate. Within the range of the amount of the reactants used, our preliminary studies showed no considerable dependence between the reaction time and the grafting extent.
Effect of initiator concentration
Effect of γ-rays dose
Graft copolymerization was studied at various doses of γ-rays by keeping other reaction conditions constant. As shown in Figure 1, the %Ge and %Gr increase with increasing in the doses of δ-rays and reach at a maximum value. Further increase of doses of γ-rays beyond 25 kGy disfavoured the grafting parameters. A relatively high dose of γ-rays may cause a reduction of %Ge and %Gr due to increase in the number of collagen free radicals terminated prior to AMPS addition. Furthermore, homopolymer formation at higher doses of γ-rays which compete with the grafting reaction for available monomer could lead to decrease in the %Gr.
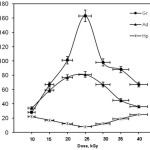 |
Figure 1: Effect of initiator concentration on the grafting parameters. Click here to View figure |
Effect of monomer concentration
The AMPS concentration was varied from 0.2 to 1.4 mol/L to study its effects on grafting parameters (Figure 2). These parameters were found to be increased by enhancement of 2-acrylamido-2-methylpropanesulfonic acid concentration from 0.2 up to 0.8 mol/L. This behavior can be attributed to the increase of monomer concentration in the vicinity of the Collagen backbone and consequent greater availability and enhancement chances for molecular collisions of the reactants. The decrease in %Gr and %Ad after a certain level of AMPS (0.8 mol/L) is probably due to preferential homopolymerization over graft copolymerization as well as increasing the viscosity of reaction medium, which hinders the movement of free radicals. Needless to say, the increase in the chain transfer to monomer molecules may be other possible reason for the diminished grafting at higher 2-acrylamido-2-methylpropanesulfonic acid concentrations. Similar observations have been reported for the grafting of ethyl acrylate onto cellulose[18-19] and methyl acrylate onto starch[20].
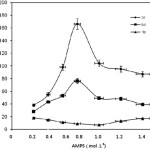 |
Figure 2: Effect of the monomer concentration on the grafting parameters.Reaction conditions: Collagen 2 wt%, γ-rays 25 kGy, temperature 55˚C, time 80 min. |
Effect of collagen concentration
The results obtained by changing the Collagen concentration for the graft polymerization are presented in Figure 3. It is evident from the figure that the %Gr and %Ad increase with increase in Collagen concentration up to 4.0 wt% and then decrease with further increment of Protein level. The initial increase may be due to the availability of more grafting sites, where Collagen can be grafted. Subsequent decrease in grafting parameters, %Gr and %Ad, in increasing collagen content more than 4.0 wt% , can be explained on the basis of increase in viscosity of the medium and a decrease in the diffusion of monomers to active sites to produce graft copolymer. This observation is in close agreement with the results obtained by Zhang and Tan [21].
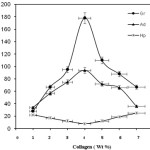 |
Figure 3: Grafting parameters as functions of Collagen concentration |
Effect of reaction temperature
To study the influence of the reaction bath temperature on the grafting parameters, the grafting of 2-acrylamido-2-methyl propan sulfonic acid onto Collagen was carried out at seven temperatures ranging from 40 to 100 oC. Figure 4 exhibits the effect of polymerization temperature on the grafting parameters. Grafting percentage (%Gr) is increased with increasing the temperature from 40 to 60 oC, and then decreased. This behavior may be related to the mobility of reactive free radical sites. Moreover, higher temperatures increase the solubility of the reactants. However, Gr was decreased as the bath temperature was raised beyond 60 oC. This can be accounted for in terms of chain radical termination at higher temperatures. Premature termination of growing chains and instability of the APS-Protein complex are presumably another reasons for reduced amount of grafting beyond 60 oC.. At higher temperatures, the rate of termination of the growing chain is increased and the monomer is volatilized out to some extent[22].
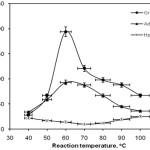 |
Figure 4: Effect of the reaction temperature on the grafting parameters. Click here to View figure |
Effect of reaction time
Grafting of 2-acrylamido-2-methyl propan sulfonic acid onto Collagen backbones was carried out at various polymerization times as shown in Figure 5. The %Gr and %Ad increased with increase in the reaction time up to 90 min and thereafter, these parameters gradually decreased. It is obvious that the longer the reaction time, the better the graft copolymerization yield. The grafting loss may be attributed to decrease of all the consuming reactants. In addition, the decreased number of available active free radical sites for grafting and the retardation of diffusion of reactants, because of the long grafted chains at the collagen surface, may be other possible reasons for the diminished grafting at longer reaction times. Similar time dependency of grafting parameters was reported by others[23].
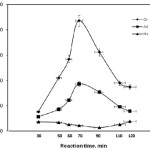 |
Figure 5: Effect of the reaction time on the grafting parameters. Click here to View figure |
Conclusions
The monomer, 2-acrylamido-2-methylpropanesulfonic acid (AMPS), can be easily graft copolymerized onto Collagen using gamma radiation as an initiator in aqueous medium. The reaction conditions were attempted to optimize for obtaining graft copolymers with higher grafting parameters. So, the reaction conditions for achieving the maximum %Gr (218) and %Ad (93) were found to be as follows: γ-rays dose, 25 kGy , AMPS 0.8 mol/L, Collagen 4 wt%, reaction temperature 60 oC, and reaction time 70 min.
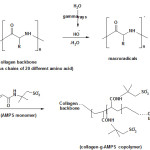 |
Scheme1: Proposed mechanistic pathway for synthesis of collagen-g-poly(AMPS) coplymer. Click here to View figure |
References
- Ibrahim, M.M.; Flefel, E.M.; El-Zawawy, W.K. J. Appl. Polym. Sci., 84, 2629-2638(2002).
- Flefel, E.M.; Ibrahim, M.M.; El-Zawawy, W.K.; Ali, A.M.Polym. Adv. Technol., 13, 541-547(2002).
- Berlin, A.A.; Kislenco, V.N., Prog. Polym. Sci.,17, 765-825(1992).
- Pourjavadi, A.; Zohuriaan-Mehr, M.J.,54, 140-147(2002).
- Doyle, C.D.,Estimating. Anal. Chem., 33 (1), 77-79(1961).
- Athawale, V.D.; Lele, V.,Starch/Starke., 52, 205-213(2000).
- D.W. Jenkins, S.M. Hudson, Chem. Rev.,101, 3245(2001).
- G.F. Fanta, Block and Graft Copolymerization, Wiley, London, (1973).
- A. Hebeish, J.T. Guthrie, The Chemistry and Technology of Cellulosic Copolymers, Springer-Verlag, Berlin, (1981).
- J. Huang, W. Xu, Appl. Surf. Sci, , 256, 3921(2010).
- S. Kalia, B.S. Kaith, J. Chil. Chem. Soc., 54, 108(2009).
- P.K. Pandey, A. Srivastava, J. Trpathy, K. Behari, Carbohyd. Polym.,65, 414(2006).
- J.M. Joshi, S.V. Kumar, Polymer, 47, 2198(2006).
- S.J. Metz, W.J.C. Van de Ven, J. Potreck, J. Membrance. Sci., 251, 29(2005).
- J.H. Ye, J.J. Dong, J.L. Lu, X.Q. Zheng, J. Jin, H. Chen, Y.R. Liang, Carbohyd. Polym.,81, 441(2010).
- I. Silva, M. Gurruchaga, I. Goñi, Carbohyd. Polym., 76, 593(2009).
- B. Singh, N. Chauhan, S. Kumar,Carbohyd. Polym.,73, 446(2008).
- G. Mino, S. Kaizerman, J. Polym. Sci.,1, 242(1958).
- G. Fu, J. Zhao, H. Yu, L. Liu, B. He, React. Funct. Polym., 67, 442(2007).
- P. Lv, Y. Bin, Y. Li, R. Chen, X. Wang, B. Zhao, Polymer, ,50, 5675(2009).
- Zhang, L.-M.; Tan, Y.-B.Macromol. Mater. Eng.,280/281, 59-65(2000).
- A. Pourjavadi, M.J. Zohuriaan-Mehr, A. Pooraghaberar, H. Hosseinzadeh, J. Polym. Mater., 21, 351(2004).
- R.E. Kirk, D.F. Othmer, ,Encyclopedia of Chemical Technology, 4rd edition, John Wiley & Sons, New York, (1992).

This work is licensed under a Creative Commons Attribution 4.0 International License.









