Microwave Assisted Synthesis of Indole Derivatives, an their Complexation Behaviour and Biological Studies
M. Asif Khan and Shamim Ahmad
Department of Chemistry, Bareilly College, Bareilly - 243 005 (India).
Indole 2-Carboxyllic acid on condensation with benzene sulfonyl hydrazide gives the condensation product – Schiff base. This has been characterized by analytical data I.R. N.M.R. spectra. The complexes of Schiff base have been prepared with metals Mn (III), V (III), Co (III), Ti (III) and Fe (III). The complexes has been characterized by elemental analyses, I,R., 1H N.M.R., electronic spectra, molar conductance and Magnetic susceptibilities. These studies suggested Octahedral geometry around the respective metal ions. The ligand and its metal complexes have been screened for their biological activity.
KEYWORDS:Microwave assisted synthesis; Indole Derivatives; Biological studies
Download this article as:| Copy the following to cite this article: Khan M. A, Ahmad S. Microwave Assisted Synthesis of Indole Derivatives, an their Complexation Behaviour and Biological Studies. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Khan M. A, Ahmad S. Microwave Assisted Synthesis of Indole Derivatives, an their Complexation Behaviour and Biological Studies. Available from: http://www.orientjchem.org/?p=23545 |
Introduction
The importance of Indole derivatives is quite evident from the number of papers, patents etc. published every year. Indole derivatives possessing antimicrobial1, antifungal2, antibacterial3 and antideprescent4activity have been reported in literature. Above mentioned few references indicate versatile nature of Indole derivatives from biological activity point of view. Use of Microwave Irradiation for synthesis of various organic compounds is also reported in literature5,6. Maganese, Vanadium and molybdenum possess a number of oxidation states and have excellent complexing property. The last two metals and their complexes exhibit biological properties7,8. Keeping in view these facts we have synthesized ligand having oxygen, nitrogen and sulphur donor and studied its structures, complexation behaviour & biological activity.
Experimental
All the chemicals used were of AR grade or equivalent purity. The chemicals used for the preparation of the ligand were Indole 2- Carboxylic acid (Sigma-Aldrich, CAS No. 1477-50-5) and benzene Sulfonyl hydrazide (LOBO India). The ligand N-(phenyl sulfonyl) – 1H-Indole -2- Carbohydrazide was prepared by reported method9. Titanium (III) Chloride was prepared in the Lab from 12% solution of Ti (III) Chloride (B.D.H.) by the reported method. All other metal salts were purchased from market and used as such.
Domestic microwave oven model M 197 DL (Samsung) was used for microwave irradiation. Melting points (m.p.) were determined on a JSGW apparatus and are uncorrected. I.R. spectra were recorded using a Perkin Elmer 1600 FT spectrometer. 1HNMR spectra were measured on a Brucker WH-500 MHz spectrometer at a Ca 5-15% solution in DMSO – d6 (TMS as Internal standard). Elemental analyses was carried out on vitro EL III Elementor Thin Layer Chromatography (TLC) was performed on Silica gel G for TLC (Merck) and spots were visualized by Iodine vapours.
Preparation of the Ligand
Indole -2- Carboxylic acid (322 mg, 2 mmol) and benzene sulfonyl hydrazide (344 mg, 2mmol) were mixed throughly. This mixture was subjected to microwave irradiation (keeping inside a microwave oven) for 2.0 Min. at 600 W power Level and reaction progress was monitored by TLC. This process was repeated three times when one of the starting materials disappeared. Crude product was washed with ethyl acetate: diethylether (5:1) (10 ml.) and the product so obtained was further purified by recrystallization from methanol to give pure product.
Preparation Of Metal Complexes
The complexes were prepared by adding the solution of metal in ethanol drop by drop to the solution of ligand till complete precipitation.
The precipitate was filtered, washed with ethanol to remove any unreacted part of either of the reactants. The precipitate was filtered and dried in Vacuum dessicator.
Anti-inflammatory activity evaluation
Anti-inflammatory activity evaluation10 was carried out using carrageenin induced paw Oedema in albino rats. Oedema in one of the kind paws was induced by injection of carrageenin solution (0.1 ml. of 1%) into planter apponeurosis. The volume of the paw was measured plethysmographically immediately after and 3.0 hour after the injection of the irritant. The difference in volume gave the amount of Oedema developed. Percent inhibition of the Oedema between the control group and compound treated groups was calculated and compared with group receiving a standard drug.
Analgesic Activity Evaluation
Analgesic was measured by writhing assay11 using mice (15-20g). Female mice were screened for writhing on day-1 by injecting intraperitonially 0.2 ml of 0.02% aqueous solution of phenylquinone. They were kept on flat surface and the number of writhes of each mouse was recorded for 20 min. The mice showing significant writhes (>10) were sorted out and used for analgesic assay on following day. The mice consisting of 5 in each group and showing significant writhing were given orally a 50 or 100 mg/kg p.o. dose of the test compounds 15 Min. prior to phenylquinone challenge. Writhing was again recorded for each mouse in a group and a percentage protection was calculated using following formula:
Protection = 100-[{No. of writhings for treated mice)/(No. of writhings for untreated mice)} x 100].
This was taken as a percent of analgesic responce and was averaged in each group of mice. Percent of animals exhibiting analgesia was determined with each dose.
Result and Discussion
The ligand and its transition metal complexes with Ti (III), V (III), Mn (III), Co (III), Fe (III) were subjected to elemental analyses where as metal and chloride were estimated gravimetrically in the lab. All this analytical data suggested 1:2, M:L stoichiometric for all the complexes.
The M.P. of the ligand and its metal complexes were determined and compared in order to find out the possibilities of formation of complexes. The M.Pts are given in Table I. The determination of molar conductance in DMSO at 10-3M dilution suggested 1:1 electrolytic nature for all the synthesized complexes.
The observed value of magnetic susceptibility was used to calculate to magnetic moment of the complexes. These values suggested paramagnetic nature for Ti (III), V(III), Mn (III), Fe (III) complexes as expected for Octahedral d1 d2 d4 and d5 complexes. The Co (III) complexes is diamagnetic in nature as expected for low spin d6 Ion. The value of magnetic moments of complexes are given in Table-I.
Table 1: Characterization of Ligand & Metal Complexes Prepared
|
Sl No |
Formula of the Ligand and Complex and Molecular Weight
|
Colour |
M.P./ D.T. 0C |
Elemental analyses |
Molar Conductance ohm-1 cm2 mole-1 |
Magnetic Moments in (B.M.) |
|||||
|
% of C |
% of H |
%of N |
% of S |
% of M |
% of Cl |
||||||
| 1a | C15H13N3SO3Mol. Wt. = 315 |
Yellow |
180 |
57.14 (56.97) |
4.12 (4.10) |
13.33 (13.22) |
10.15 (10.0) |
– |
– |
– |
– |
| 1b | [C30H24N6S2O6 Ti]ClMol. Wt. = 711.5 |
Yellow |
235 |
50.59 (50.50) |
3.37 (3.35) |
11.80 (11.76) |
8.99 (8.90) |
6.74 (6.71) |
4.98 (4.97) |
61 |
1.71 |
| 1c | [C30H24N6S2O6 V]ClMol. Wt. = 714.5 |
Yellow |
253 |
50.38 (50.36) |
3.35 (3.34) |
11.75 (11.72) |
8.95 (8.90) |
7.12 (7.0)
|
4.96 (4.93) |
64 |
2.94 |
| 1d | [C30H24N6S2O6 Mn]ClMol. Wt. = 718.5 |
Brown |
261 |
50.10 (50.0) |
3.34 (3.33) |
11.69 (11.67) |
8.90 (8.88) |
7.65 (7.63) |
4.94 (4.91) |
68 |
5.40 |
| 1e | [C30H24N6S2O6 Fe]ClMol. Wt. = 719.5 |
Dark Brown |
268 |
50.03 (50.0)
|
3.33 (3.32) |
11.65 (11.62) |
8.89 (8.85) |
7.78 (7.75) |
4.93 (4.90) |
73 |
5.24 |
| 1f | [C30H24N6S2O6 Co]ClMol. Wt. = 722.5 |
Yellowish Brown |
271 |
49.82 (49.75) |
3.32 (3.31) |
11.62 (11.60) |
8.85 (8.82) |
8.16 (8.06) |
4.91 (4.89) |
79 |
Diamagnetic |
Figures in parenthesis are observed values.
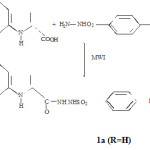 |
Scheme 1 Click here to View scheme |
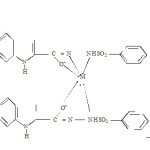 |
Scheme 2 Click here to View scheme |
(1b, 1c, 1d, 1e and 1f)
Where M = Ti(III), V(III), Mn (III), Fe (III) and Co (III
Electronic Spectra
The electronic spectrum of the complex of Ti (III) exhibits a single broad band at 19230 Cm-1 assignable to
2t2g 2Eg transition for Oh symmetry12. The electronic spectrum of complex V (III) exhibits band at 1600 Cm-1 with a shoulder at 20,500 Cm-1. The low energy band has been assigned to 2A1g→3A2g while the high energy band may be due to 2A1g→3T2g(P) transition. These bands are characteristic of Octahedral geometry13.
The electronic spectrum of Mn (III) complex showed an intense and sharp charge transfer band at 22000 Cm-1 and a spin allowed d-d transition band 5Eg→5T2g at 18500 Cm-1. This broad band occuring at lower frequency with increased intensity indicates the lowering of symmetry from Octahedral Configuration14.
The electronic spectrum of the complex of Fe (III) exhibited there bands at 11235, 21470 and 27780 Cm-1 assignable to 6A1g→4T1g, 6A1g→4T2g and
6A1g→4Eg transitions respectively. These transitions are characteristics of Octahedral Fe (III) complexes15.
The electronic spectrum of Co (III) complex displays bands at 15110, 21095 and 23370 Cm-1 assignable to 3A1g→3T2g, 1A1g 1T1g and 1A1g→1T2g transitions respectively. These are similar to those reported for other six coordinated Co (III) complexes16.
I.R. SPECTRA
The I.R. spectrum of the ligand shows bands at 3370 and 3310 Cm-1 due to the presence of two N-H groups. The bands at 1640, 1450, 1380, 890 and 1000 cm-1 are assign to n (C=0), amide I [b (NH) + n (CN)] amide-II [n (CN) + b (NH)], n (C=S) and n (N-N) modes respectively17.
In the I.R. spectra of complexes bands due to n N-H, and n (C=0) are absent. But new band appears at 1600 Cm-1 due n (N=C) of NCO suggesting removal of N-H proton via enolization and bonding of resulting enolic oxygen with the metal ion. Furthermore, the amide I and II band and n (N-N) band in the free hydrazide undergo positive shifts of 30-40 Cm-1 suggesting involvement of both hydrazinic group in bonding in addition to the enolic oxygen. Thus the ligand is behaving in univegative tridentate manner.
The I.R. spectrum of the ligand shows ring vibration of indole moiety at 1625, 1565 and 1520 Cm-1 which remain unaltered in the I.R. spectra of complexes excluding the possibility of involvement of the N atom of indole in bonding.
The 1H-NMR spectrum of the ligand shows signals at 89.66 ppm due to NHC(o) which disappear on D2O exchange suggesting removal of NHC(o) proton via enolization. Benzene ring proton appears at 87.63, 7.33 and 7.16 ppm18.
Conclusion
On the basis of above mentioned studies an octahedral geometry may be proposed for all the synthesized complexes.
Biological Studies
Compounds 1a and 1b, 1c, 1d, 1e and 1f at 100 mg./kg. P.O. were tested for anti-inflammatory activity in the Carrageenin induced paw edema model10 and the results are summarized in Table-II. Compounds 1a, 1b, 1c, 1d, 1e and 1f at 100 mg/kg P.O. were screened for analgesic activity using phenylquinone writhing assay11 and the results are reported in Table-II.
Table 2: Anti-Inflammatory and Analgesic activity evaluation
|
Compound |
Dose mg/kg P.O. |
Anti-Inflammatory activity % |
Dose mg/kg P.O. |
Activity
|
|
1a |
100 |
0.0 |
100 |
50 |
|
50 |
25 |
|||
|
1b |
100 |
0.0 |
100 |
60 |
|
1c |
100 |
0.00 |
100 |
20 |
|
1d |
100 |
0.0 |
100 |
50 |
|
1e |
100 |
0.0 |
100 |
20 |
|
1f |
100 |
0.0 |
100 |
25 |
P.O. = from Latin word per OS (means by month)
References
- Saundane A.R., Sharma P. M.V. and Badiger J., Indian J. Hetrocyclic Chem., 14, 307, (2005).
- Agarwal A., Agarwal S.K., Shukla P.K. & Khan Z.K., Indian IN 183635; Chem. Abstr. 141, 260731 (2004).
- Palluotto F., Campagna F., Carrotti A., Ferappi M., Rosato A. and Vitali C., Farmaco, 57, 63 (2002).
- Stack G.P., Tran M. and Bravo B. A., PCT Int Appl WO 2002088146; Chem. Abstr. 137, 353044 (2002).
- Varughese D.J. Manhas M S and Bose AK, Tetrahedron Lett. 47, 6795 (2006).
- Bose A.K., Ganguly S.N., Manhas M.S., Guha A. and Pombo-Villars E., Tetrahedron Lett., 47, 4605 (2006).
- Sondhi S.M., Dinodia M. and Kumar A. Bioorg, Med. Chem. 14, 4657 (2006).
- Sondhi S.M., Dinodia M., Singh J. and Rani R, Current Bioactive Compounds 3, 91, (2007).
- Sham M. Sondhi, Shubhi Jain, Reshma Rani and Ashok Kumar, I.J.C. 46-B, 1848-1854 (2007).
- Winter C.A., Risley E.A. and Nuss GW, Proc. Soc. Exp. Biol. Med, 111, 544 (1962)
- Singh P.P., Junnarkar AV, Seshagiri Rao C, Verma R.K. and Shridhar D.R., Meth and find exptl. Clini Pharmacol, 5, 601 (1983).
- Mohammad Azim and Shamim Ahmad, Vol. 27, No. (2), 673-677 (2011).
- Rahul Kumar Rastogi, Poonam Garg and Shamim Ahmad, Asian Journal of Chem. Vol. 21, No. 8, 6144-6148 (2009).
- G.S. Bhadange, R.B. Mohod and A.S. Aswar Indian Journal of Chemistry Vol. 40 A, PP 1110-1113 (2001).
- Patel MM, Patel MR, Patel MN and Patel RP, Indian J. Chem. 20A, 6623 (1981).
- Choudhary C.K., Choudhary Ratan K, and Mishra L.K., J. Indian Chem. Soc. 80, 693-695 (2003).
- Singh M.K. and Kushawaha, I.J.C., Vol. 43-A, 333-336 (2004).
- Sham M. Sondhi, Shubhi Jain, Reshma Rani and Ashok Kumar, I.J.C. 46-B, 1848-1854 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









