Kinetics of Oxidation of Allyl Alcohol By Cr(Vi)
K. G. Sekar1 and V. Palanivel2
1Department of Chemistry, National College (Autonomous),Tiruchirappalli - 620 001 (India). 2Department of Chemistry, Periyar EVR College, (Autonomous), Tiruchirappalli - 620 023 (India).
The kinetics of oxidation of allyl alcohol by quinaldinium chlorochromate is investigated in 40 % acetic acid – water (v/v) medium. The order with respect to oxidant and hydrogen ion concentration is found to be one and fractional with respect to substrate. The rate of the reaction is decreases with the decrease in the dielectric constant of the medium. The rate of the reaction has negligible effect with the increase of ionic strength. The reaction does not induce the polymerization of acrylonitrile. From the kinetic data obtained, the activation parameters have been calculated and a plausible mechanism has been proposed.
KEYWORDS:Kinetics; Mechanism; Oxidation; Allyl alcohol; Quinaldinium Chlorochromate
Download this article as:| Copy the following to cite this article: Sekar K. G, Palanivel V. Kinetics of Oxidation of Allyl Alcohol By Cr(Vi). Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Sekar K. G, Palanivel V. Kinetics of Oxidation of Allyl Alcohol By Cr(Vi). Available from: http://www.orientjchem.org/?p=23530 |
Introduction
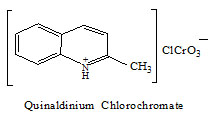
Quinaldinium chlorochromate (QnCC) has been used as a mild and stable oxidizing agent in synthetic organic chemistry (1). The structure of QnCC is given below,
Allyl alcohol (prop-2-en-1-ol) is an organic compound with the structural formula CH2=CHCH2OH. It is a water soluble and colourless liquid. Allyl alcohol is used as a raw material for the production of glycerol and also used as a precursor to many specialized compounds. The kinetics and mechanism of oxidation of allyl alcohol by various oxidants have been reported (2-11). The kinetics of oxidation of allyl alcohol by QnCC has not been reported by investigation. The kinetics of oxidation of allyl alcohol by QnCC in aqueous acetic acid medium has been investigated and also the mechanism and rate law has been derived.
Experimental
Materials and Methods
Preparation of quinaldinium chlorochromate
Chromium trioxide (10.0 g) was dissolved in 6 M hydrochloric acid (18.4 ml) and then it was cooled to 0° C (ice-water bath). Quinaldine (13.9 ml) was added to chromium trioxide solution drop-wise during 30 minutes. The reaction mixture was cooled for 2 h. The resulting product was filtered off and washed with ether (4´50 ml) affording Quinaldinium chlorochromate (10 g) as yellow solid m.p. 139° C, yield 72%. From the IR spectral data, it was confirmed.
Allyl Alcohol
Allyl alcohol was distilled and the fraction collected at 97ºC was used.
Acetic Acid
Glacial acetic acid (AR) (2 litre) was partially frozen and about 1 litre of the liquid was removed. The residue was melted and refluxed with chromium trioxide (30 g) for 4 h and fractionally distilled (12). The distilled portion was collected between 116-118◦C, partially frozen and about half of the acid was discarded as liquid. The remaining residue was melted and fractioned again after treating with chromium trioxide (30 g). The boiled fraction was collected 116-118° C and kept in brown bottles.
All other chemicals were used as AR grade. Triply distilled water was used for the preparation of solutions.
Kinetic measurements
The reaction was carried out under pseudo – first order conditions [Allyl] ≫ [QnCC] in 40 % (v/v) aqueous acetic acid medium containing perchloric acid. The course of the reaction was followed spectrophotometically at 470 nm for upto 80% of the reaction and the pseudo – first order rate constants k1 were computed from the linear plots of log absorbance versus time by the least square method, and reproducible within ±1%
Stoichiometry and Product analysis
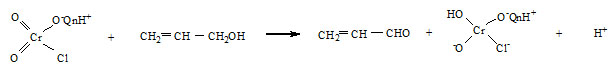
Reaction mixture containing an excess of the oxidant over allyl alcohol were kept at room temperature in the presence of perchloric acid for 24 h. Estimation of the unchanged oxidant showed that one mole of allyl alcohol consumed one mole of the oxidant. The product acrolein , was identified by IR spectra and the reaction proceeds quantitatively according to the equation,
Results and Discussion
Effect of varying quinaldinium chlorochromate
The kinetic data reveals that the rate of oxidation of allyl alcohol by quinaldinium chlorochromate is found to be first. The plot of log absorbance versus time is linear indicating that the order with respect to quinaldinium chlorochromate is unity. The pseudo-first order rate constants were found to be independent of initial concentrations of quinaldinium chlorochromate.(Table 1)
Table 1: Rate constants for the oxidation of Allyl alcohol by QnCC at 40ºC
|
[Allyl]×102 (M) |
[QnCC]×103 (M) |
[HClO4]×101 (M) |
[NaClO4]×103 (M) |
AcOH-H2O (%v/v) |
kobs×104 (s-1) |
|
2.0 – 10.0 |
7.2 |
1.0 |
—- |
40 |
4.87-6.25 |
|
4.0 |
3.6-18.0 |
1.0 |
—- |
40 |
5.47-5.99 |
|
4.0 |
7.2 |
0.5-2.5 |
—- |
40 |
3.39-12.88 |
|
4.0 |
7.2 |
1.0 |
0.0-7.2 |
40 |
5.47-5.70 |
|
4.0 |
7.2 |
1.0 |
—- |
30-50 |
5.01-6.17 |
Effect of varying allyl alcohol
At constant [QnCC], the rate constants are increased with the increase in the concentrations of the allyl alcohol( Table 1). A plot of log k1 versus log [substrate] with a slope was 0.15. It was further supported by the fact that the double reciprocal plot of kobs versus [substrate] gave a straight line with a definite intercept indicating the Michaeli’s-Menten type of this reaction (Figure. 1).
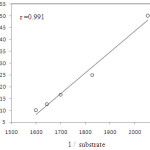 |
Figure 1: Plot of 1/k versus 1/substrate |
Effect of varying Hydrogen ion concentration
The effect of acidity was studied by varying the concentrations of perchloric acid and the rate constants were increased with the increase in the concentrations of perchloric acid (Table 1). The reaction was found to be first order in hydrogen ion as evidenced by the unit slope of the plot of log k1 versus log [H+]. (Figure. 2)
![Figure 2: Plot of log k1 versus log [H+]](http://www.orientjchem.org/wp-content/uploads/2012/06/vol28_iss2_sek_kin_fig2-150x150.jpg) |
Figure 2: Plot of log k1 versus log [H+] |
Effect of varying solvent composition
Increase in the percentage of acetic acid of the medium increases the rate of the oxidation process (Table 1). This is normally observed in the oxidation reactions of Cr(VI). Further correlation of log kobs with the reciprocal of the dielectric constant of the medium give linear plot (Figure. 3) suggesting an ion- dipole interaction (13-15) between allyl alcohol and quinaldinium chlorochromate.
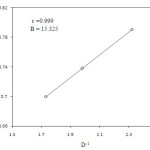 |
Figure 3: Plot of log k1 versus D-1 |
Effect of varying ionic strength
The rate constants were constant when the concentrations of the sodium perchlorate increases. There was no appreciable change in the rate with the change of ionic strength (Table 1) indicating the involvement of neutral molecules in the rate determining step (16).
Effect of added acrylonitrile
The reaction does not induce polymerization of acrylonitrile indicating the absence of free radical pathway (17).
Effect of varying manganous sulphate
The reaction was followed with the varying concentrations of Mn2+ ions keeping all the other factors constant. There was an appreciable decrease (18 – 19) in the rate with the increase of the concentrations of Mn2+ ions confirms the involvement of two electron transfer process in the reaction (Table 2).
Table 2: Effect of varying manganous sulphate
[QnCC] = 7.20×10-3 mol dm-3 [H+] = 10.00×10-2 mol dm-3
[Allyl alcohol] = 4.00×10-2 mol dm-3 AcOH-H2O = 40: 60 (%)
Temperature = 313 K
|
[MnSO4] ×104 ( M ) |
k1×104 s-1 |
| 0.00 | 5.48 |
| 1.80 | 5.00 |
| 3.60 | 4.96 |
| 5.40 | 4.55 |
| 7.20 | 4.00 |
Effect of varying temperature
The reaction has been carried out at four different temperatures 303 K, 313 K, 323 K and 333 K respectively, keeping all other factors constant (Table 3) .The thermodynamic parameters have been computed from the linear plot of ln (k2/T) versus 1/T of Eyring’s equation (20) (Figure. 4)
∆H# = 22.09 k J mol-1
∆S# = -210.29 J K– 1mol-1
∆G# = 96.33 k J mol-1 at 313 K
Ea = 24.69 k J mol-1 at 313 K
Table 3: Effect of varying temperature
[QnCC]=7.20×10-3 mol dm-3 [H+] =10.00×10-2 mol dm-3
[Allyl alcohol] = 4.00×10-2 mol dm-3 AcOH-H2O = 40:60 (%)
|
Temperature K |
k1 × 104 s-1 |
|
303 |
4.24 |
|
313 |
5.47 |
|
323 |
7.60 |
|
333 |
10.17 |
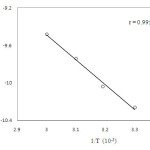 |
Figure 4: Plot of ln k2/ T versus 1/T |
Mechanism and rate law
From the above observations, it is clear that the reaction is showing unit order with respect to oxidant and [H+] ion and fractional order with respect to substrate. And the following mechanism is proposed for the oxidation of allyl alcohol by quinaldinium chlorochromate.
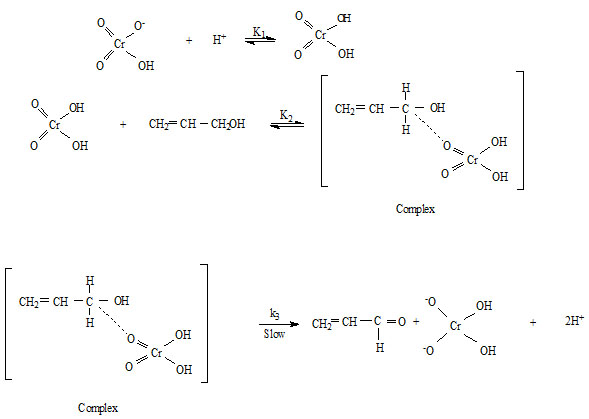
Rate law
These above observations suggest that the rate law can be shown as
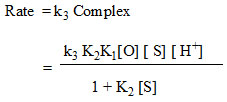
Conclusion
The oxidation of allyl alcohol by quinaldinium chlorochromate in aqueous acetic acid medium leads to the formation of complex and finally gives the product. The reaction follows simple order kinetics.
Acknowledgement
The authors are thankful to the authorities of National College (Autonomous), Tiruchirappalli for providing facilities.
References
- Nebahat Degirmenbasi and Beytiye Ozgun, Monatshefte furchemie., 135 ,407(2004).
- Ganapthy. K and Vijiyan. B, Proc. Indian Acad.Sci., 87A, 215(1897).
- Babaeva. L. G, Bogatkov. S. R, Kruglikova R. I and Uhkouskii. B. V, Zh. Org. Chim.,10, 250(1974).
- Yathirajan. H. S, Rangaswamy, and Mahadevappa.D. S, J. Indian.Chem.Soc., 56,421(1979).
- Srivastava. S. P, Gupta. V. K, Gupta, J. C and Maheswari.M. K, J. Indian. Chem. Soc.,57,797(1980).
- Dwivedi. R. K, Verma. M, Kumar. P and Behari.K, Tetrahedron.,39, 819(1983).
- Herlihy Kevin.P, Aust. J. Chem., 36,206(1983).
- Yachkor. D, Likhteror. A. I and Elis.V, Zh. Org. Khim., 20, 913(1984).
- Veechha. N. D and Pandey.A. K, J. Indian Chem. Soc., 63, 670(1986).
- Gantla Sreelatha, Mulakaluri Prasad Rao, Bangalore Sethuram and Tangeda Navaneeth Rao, Transition Metal Chemistry., 1, 31(1990).
- Subbiah Meenakshisundaram and Ramanathan Sockalingam, Collect. Czech. Chem. Commun., 66, 877(2001).
- Kohler. E. P, Gilman and Blatt.A. H, Organic synthesis, John wiley, Newyork,78(1956).
- Quinlan, and Amis. E. S, J. Am. Chem. Soc., 77, 4187(1955).
- Amis. E. S, Solvent Effects on Reaction Rates and Mechanism, Academic Press, New York, 42(1966).
- Priya. V, Balasubramaniyan. M and Mathiyalagan. N, J. Chem. Pharm. Res., 3(1),522(2011).
- Singh. R. A, Kamini Singh, Singh. S. K, J. Chem. Pharm. Res., 2(3), 684(2010).
- Vellaisamy. M, Suryakala. K and Ravishankar.M, J. Chem. Pharm. Res., 3(5),678(2011).
- Firoz Ahmad, Ritu Singh and Abbas Siddiqui.M, J. Chem. Pharm. Res., 4(1), 608(2012).
- Rajalakshmi. K, Ramachandramoorthy, T and Srinivasan. S, J. Chem. Pharm. Res.,4(1), 894(2012).
- Eyring.H, J. Chem. Phys., 33, 107(1935).

This work is licensed under a Creative Commons Attribution 4.0 International License.









