Isoxazole Functionalized Crosslinked Resin Complexes of Transition Metals as Polymeric Catalysts for Hydrogen Peroxide Decomposition
G. Rathika Nath1 and K. Rajesh2
1Department of Chemistry, Sree Ayyappa College for Women Chunkankadai, Nagercoil, (India). 2Department of Chemistry, University College,Trivandrum, (India).
Crosslinked functionalized polymers are highly efficient in immobilizing transition metal ions. The activity of Fe(III), Co(II), Ni(II) and Cu(II) complexes of polystyrene bound Isoxazole resin have been tested towards the decomposition of hydrogen peroxide. The decomposition reaction followed a first order dependence on the concentration of both the substrates and the catalyst. The metal complexes having less cross linking at the backbone and ring-activating group near the active site show high catalytic efficiency.
KEYWORDS:Polymer Supported Ligands; Metal Complexes; Poly-Styrene Supported Ligands; Isoxazole Ligands; Hydrogen Peroxide
Download this article as:| Copy the following to cite this article: Nath G. R, Rajesh K. Isoxazole Functionalized Crosslinked Resin Complexes of Transition Metals as Polymeric Catalysts for Hydrogen Peroxide Decomposition. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Nath G. R, Rajesh K. Isoxazole Functionalized Crosslinked Resin Complexes of Transition Metals as Polymeric Catalysts for Hydrogen Peroxide Decomposition. Available from: http://www.orientjchem.org/?p=23511 |
Introduction
Polymer bound metal catalysts have found many applications in modern synthetic organic chemistry. In functionalized cross-linked polymers with transition metal complexes acting as catalysts, the polymer matrices can play a role of mere support for catalytic sites thus facilitating the work-up of the reaction products and also can directly control the reaction kinetics which depends on the loading and distribution of catalytic sites along the polymer backbone. The main advantages of using macromolecular chiral catalysts are easy recovery and reuse, simplified reaction work-up and a potential to be carried out in a flow reactor or flow membrane reactor for continous production.1,2
But the applications of majority of polymer supported metal complex catalysts based on macroporous polystyrene-based resin has been limited due to two main reasons.3, 4 The first major reason is the catalyst’s instability arising from the leaching of metal complex from the support. The second major reason arises from an inherent belief that the normal polymer supports would be too thermooxidatively unstable to be used in anything other than mild oxidation processes. An upper operating temperature limit of above 200°C is usually envisaged. From thermogravimetric studies, isoxazole containing polymers are identified as a potentially important and versatile polymer support due to their exceptional thermal stability.
The catalytic properties of metal complexes derived from isoxazole containing ligands have been studied.
Decomposition of Hydrogen Peroxide
One of the main standard reaction often employed to determine the catalytic activity of a metal complex is the decomposition reaction of Hydrogen peroxide.5-9 Metal ions as such or their complexes are observed to possess enzyme like activity. The metal ion catalyzed disproportionation of H2O2 has received much attention which is mainly stimulated by the natural occurrence of a heme-iron enzyme called catalase which enhances the decomposition dramatically.10
2H2O2 → O2 + 2H2O
The reaction is also catalyzed by Cu (II) complexes11-13 provided the coordination sphere is accessible for H2O2 and HOO–. The peroxides are cheap and readily available on a large scale from auto-oxidation of tertiary hydrocarbons. There has been and continues to be a lot of activity directed towards developing potentially selective catalysts for the decomposition of peroxides.
The Hydrogen peroxide decomposition reaction usually obeys first order kinetics and the study is important in understanding oxygen transport in polymer metal chelates.
Experimental
Preparation of the Polymer Bound Isoxazole Metal Complexes
The synthesis of the polystyrene bound isoxazole complexes involves the reported method.14
Reagents and Materials
All reagents used were of Analar grade. H2O2 (40 % v/v) was used as such from NICE chemicals.
Decomposition of hydrogen peroxide
Polymer supported isoxazole linked metal complex (100 mg) was shaken with hydrogen peroxide solution (0.4 V, 100 ml). Taking aliquot amounts and titrating against standard KMnO4 (0.02N), the amount of H2O2 remaining after definite period of time were determined. The course of decomposition was followed over a period of 3 hours. A blank experiment was also conducted side by side. Similar experiments were carried out by varying the conditions such as temperature, amount of catalyst and the concentration of H2O2. The effect of Macromolecular factors like the degree of cross-linking and structural environment on the decomposition reaction were also studied by carrying out similar experiments.
Results and Discussion
A definite amount of metal complex was shaken with H2O2 of known volume and strength. The amount of H2O2 decomposed was followed by withdrawing a known amount of H2O2 at definite intervals of time and titrating it against standard KMnO4. The rate constant K of the decomposition pattern of H2O2 using different polymeric catalysts, calculated using the first order equation
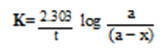
where a is the initial concentration of the reactant and x is the amount reacted in time t, are given in table 1. The activation energies of catalyzed and uncatalyzed reactions were compared to get an idea of their relative efficiencies. The activation energies were calculated using the Arrhenius equation,
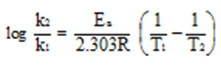
All the complexes, except that of Co (II) were found to increase the rate of decomposition, proving their catalytic activity. The poor activity of Co (II) complexes of the ligand can be attributed to its assigned geometry, which do not favour decomposition reaction. Among the four metal complexes, Cu (II) complexes show the highest catalytic activity.
Effect of various factors on decomposition
In order to establish the relation between rate of decomposition and various factors such as substrate concentration, amount of catalyst, temperature, degree of cross-linking and structural environment of the ligand, studies on aqueous hydrogen peroxide solutions at varying conditions were carried out.
Influence of substrate concentration
The decomposition patterns of aqueous H2O2 solutions of varying concentrations were studied in presence of a definite amount of catalyst and the rate constant for each case are given in table 2.The rate of the decomposition reaction was found to be increasing with increase in substrate concentration.
Effect of amount of catalyst
The decomposition patterns of aqueous H2O2 solutions of definite volume and concentration were studied in presence of varying amount of catalysts after thermostating at 293 K and the rate constant for each case are given in table 3. The results obtained prove the linear relationship between rate constant and the amount of catalyst.
Influence of degree of cross-linking of polymer backbone
The study of the effect of degree of cross-linking of the polymer supported metal complexes on the decomposition pattern has been studied and the results are tabulated in table 4. A gradual decrease in catalytic efficiency with increase in the degree of cross-linking was noticed. With increasing degree of cross-linking, the hydrophobicity of the polymer increases which in turn decreases the accessibility of reactive groups. Thus the reactive sites in highly cross-linked systems will not be readily available to the low molecular weight species.
Influence of structural environment of the ligand
Polymer supported isoxazole complexes with different substituents groups such as phenyl, 4’-methoxy phenyl, 4’-methyl phenyl, 4’-chloro phenyl and 4’-nitro phenyl were used as catalysts and the rate of decomposition was estimated for each case after a fixed time. The results are tabulated in table 5. Studies revealed that the methoxy substituted complexes possessed higher metal intake than others. This trend was noticed because of their direct relationship on their activity on the decomposition reaction. The inductive and the mesomeric effect of the substituents groups influence the electron delocalisation over the chelating ligand. Electron availability of the coordinating sites, in turn, determines the basicity of the ligand function. Methoxy and methyl groups, being electron donors, increases the basicity which promotes the electron transfer process between the metal complex and the substrate. On the other hand, the chloro and nitro groups decrease the basicity of the functional groups due to their electron withdrawing nature.
Influence of temperature
The variation in rate of decomposition of aqueous H2O2 solutions of definite concentrations thermostated at different temperatures were studied with definite amount of catalysts. The rate constant values when temperature is varied from 20°C to 60°C are given in table 6. From the results it is clear that the rate constants bear a linear relationship with temperature. From kinetic studies, it is clear that the reaction follows first order kinetics. The activation energies of complex catalyzed decomposition were found to be much less than the uncatalyzed reaction, proving the high efficiency of catalysts on increasing the rate of the reaction.
Discussion
Metal ions and their complexes can bind molecular oxygen rapidly and reversibly quite similar to metalloporphyrins in a living body . Polymer metal complexes show specific behaviour in the binding of small molecules since the polymer backbone affects the reaction to a large extent.
The action of complex carriers in the reaction is the picking up of oxygen from one end and diffusing inside as the oxygenated complex and finally transferring oxygen to the other side. The decomposition reaction is accompanied by the liberation of molecular oxygen, with the reduction of metal ions in the complex to a lower oxidation state resulting in the shrinking of the polymer. This observation is dependant on the geometry of the polymer structure. A transient complex with a new geometry which is capable of accepting an electron is formed due to the stretching of the metal-ligand bond, which causes lowering of the activating energy and enhancement of catalytic activity. The energy required to rearrange the complex structure is the activation energy of the electron transfer15.
Conclusion
Marked activity towards the decomposition of hydrogen peroxide was found with Fe(III), Co(II), Ni(II) and Cu(II) complexes of polystyrene bound isoxazole resin complexes. The reactions showed first order dependence on the concentration of both the substrates and the catalyst. The metal complexes having less cross linking at the backbone and ring-activating group near the active site showed high catalytic efficiency. This is only a preliminary study which shows that crosslinked polymeric isoxazole functionalized metal complexes have marked activity towards Hydrogen peroxide decomposition. The main utility of this knowledge is that the isoxazole functionalized resin complexes with various metals can be used to catalyse many organic reactions in which nascent oxygen is utilized. Thus this property can be applied in further studies where oxidation by nascent oxygen is used like epoxidation reactions.
Acknowledgement
The authors wish to acknowledge funding by the UGC
References
- Itsuno S., Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis. Wiley-VCH (2011)
- Jacobsen E.N., Pfaltz A., Yamamoto Y., Comprehensive Asymmetric Synthesis. Springer, (1999)
- Tokoyamo K., Nishizawa M., Kimura T., Suzuki T.M., Bull.Chem.Soc.Jpn. 58: 3271 (1985)
- Sherrington D.C., Simpson S., J.Catal. 131: 115 (1991)
- Rathika Nath G., Rajesh K., Orient. J. Chem., 27 (3), 1215-1220 (2011)
- Nina Kann, Molecules 15: 6306-6331 (2010)
- Pepper K.W., Paisley H.M., Young,M.A., J.Chem.Soc. 4097 (1953)
- Sreekala R., Yusuff K.K.M., Ind.J.Chem, 34A: 994 (1995)
- Joseph D.L., Herve G., Environ.Sci.Technol. 33: 2726 (1999)
- Jones P., Wilson I., Met.Ions biol.Syst. 7: 185 (1978)
- Sigel H., Wyss K., Eischer B.E., Prijs B., Inorg.Chem., 18: 1354 (1979)
- Oishi N., Takeuchi M., Nishida Y., Kida S., Polyhedron 3: 157 (1984)
- Nath M., Kamaluddin, Cheema J., Ind.J.Chem. 32A: 108 (1993)
- Rathika Nath G., Radhakrishnan T., Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry 35: 491–498 (2005)
- Tsuchida E., Nishide H., Nishiyamam T., Macromol.Chem. 175: 3047 (1974)

This work is licensed under a Creative Commons Attribution 4.0 International License.









