Determination of Chloropyrifos in Fruits and Vegetables by Using Analytcal Techniques
N.V.S. Venugopal*, B .Sumalatha and Srinivasa Rao Bonthula
Department of Chemistry, G.I.T, GITAM UNIVERSITY, Rushikonda, Visakhapatnam - 530 045 (India).
Highly sensitive spectrophotometric and gas chromatographic methods were developed for the determination of chloropyrifos.Alkaline hydrolysis of chloropyrifos to 1,2,4-trichloropyridine was the basis followed by coupling with congored in presence of nitric acid.The absorption maxima of blue color formed at 800-900C was formed at 605nm.Beer’s law was obeyed in the range of 0.5-5.7ppm.The standard deviation was found to be ± 0.005.The method was free from other pesticide interferences.The method was applied to various fruits and vegetables procured at various agricultural fields at sabbavaram area,Visakhapatnam,India and found satisfactory.Keeping in view of extensive use of pesticides on fruits and vegetables damaging the ecological balance. Determination of pesticide residue in The various vegetables collected are cauliflower, potato, spinach, and fruits suchas pinkapple,grapes etc were analysed by using Gas –chromatography with electron capture detector ( GC-ECD). Residues were extracted with hexane,dichloromethane and ethyl acetate (1:1% V/V).Clean up procedure was carried by applying solid phase extraction column. The recoveries of chloropyrifos pesticide in fruits and vegetables selected were in the range of 80 to 98% fortified at 0.5mg/Kg.Some grape samples showed above the limit of quantification.
KEYWORDS:Chloropyrifos; Congored; Spectrophotometer; GC-ECD; Fruits; Vegetables
Download this article as:| Copy the following to cite this article: Venugopal N. V. S, Sumalatha B, Bonthula S. R. Determination of Chloropyrifos in Fruits and Vegetables by Using Analytcal Techniques. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Venugopal N. V. S, Sumalatha B, Bonthula S. R. Determination of Chloropyrifos in Fruits and Vegetables by Using Analytcal Techniques. Available from: http://www.orientjchem.org/?p=23509 |
Introduction
Chloropyrifos is a crystalline organophosphate insecticide.The IUPAC name of chloropyrifos is O, O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate and with molecular formula C9H11Cl3NO3PS. Chlorpyrifos is moderately toxic and chronic exposure has been linked to neurological effects, developmental disorders, and autoimmune disorders. Chlorpyrifos is manufactured by reacting 3,5,6-trichloro-2-pyridinol with diethylthiophosphoryl chloride[1]. Chlorpyrifos is registered only for agricultural use, where it is “one of the most widely used organophosphate insecticides”, according to the United States Environmental Protection Agency (EPA). The crops with the most intense chlorpyrifos use are cotton, corn, almonds, and fruit trees including oranges and apples, It is produced via a multistep synthesis from 3-methylpyridine.
Chlorpyrifos is an organophosphate, with potential for both acute toxicity at larger amounts and neurological effects in fetuses and children even at very small amounts. Recent research indicates that children exposed to chlorpyrifos while in the womb have an increased risk of delays in mental and motor development at age 3 and an increased occurrence of pervasive developmental disorders such as ADHD[2]An earlier study demonstrated a correlation between prenatal chlorpyrifos exposure and lower weight and smaller head circumference at birth[3]. A study of the effects of chlorpyrifos on humans exposed over time showed that people exposed to high levels have autoimmune antibodies that are common in people with autoimmune disorders. There is a strong correlation to chronic illness associated with autoimmune disorders after exposure to chlorpyrifos[4].
The immediate health hazard from air born chlorpyrifos in the examined houses was negligible, but the findings suggest that it is necessary to monitor chemicals which may contaminate indoor air and to assess the risk of prolonged exposure to such chemicals. The measuring of urinary metabolite TCP of chlorpyrifos via biological monitoring would be useful, allowing comprehensive evaluation of the exposure to chlorpyrifos in indoor air[5].Chloropyrifos is used for termite control in construction,forestry and field crops.
Numerous instrumental methods have been described for thre detection/determination of chloropyrifos generally analysed by spectrophotometry [6-7], thin layer chromatography (TLC)[8], and GC-MS[9], liquid chromatography,mass spectrometry[10], and Gas chrmatography[11].The purpose of this study was to develop a spectrophotometric method and an analysis scheme for determination of chloropyrifos in cauliflower, potato, spinach, and fruits suchas pinkapple,grapes etc by GC-ECD.
In this paper the author extracted endosulfan pesticide in fruits and vegetables using hexane,hexane-diehtyl ehter,hexane and dichloromethane as solvents,clean up on solid phase extraction(SPE) and determined on Gas Chromatography with electron capture detector(GC-ECD).
Experimental
Chemical and reagents
A JASCO (Model UVIDEC-610 UV-VIS Spectrophotometry with 1cm matched quartz
cuvettes was used for all absorbance measurements.Systonics PHmeter(model 331) is used.
All chemicals and reagents used are AnalR grade.The organic solvents like dichloromethane, ethyl acetate and hexane used were HPLC grade and purchased from E Merck. Technical grade pesticide standards were used for standardisations. Anhydrous sodium sulphate (AR) from E Merck used for residue extraction.
Extraction and clean up
Each vegetable was chopped into small pieces.All vegetables and fruits were collected from agricultural fields near sabbavaram area,Visakhapatnam District,India. A representative sample (50gm) was taken with 5-10gm anhydrous sodium sulphate in blending machine to make fine paste. The sample was extracted with 100 ml of ethyl acetate,hexane or dichloromethane on mechanical shaker for one hour, extract was filtered, concentrated up to 5 ml on rotary evaporator and finally injected into GC-ECD. The clean-up of chlorpyrifos was performed out by using column chromatography.
packed with Florisil.The extract was eluted with ethyl acetate,hexane or dichloromethane.
Elute was concentrated to 5-10 ml on rotary evaporator.
Sampling: Several samples of Water, Fruits and Vegetables were collected from Agricultural fields in Sabbavaram,Visakhapatnam district.Samples of one kilogram Grapes,pine apple (fruits) and Carrot,Cabbage, (vegetables) were procured and kept in refrigerator till analysis.
Procedures
Spectrophotomeric method
1mL of alcoholic potassium hydroxide was added to an aliquot of working standard of chloropyrifos(0.5-6.0 µg/mL).2.0mL of 0.1% congored and 2mL of 1:1 nitric acid are added to give pale blue color. The solution was kept aside for 5 min before taking absorbance and absorbance was measured at 605 nm against reagent blank. The absorbance corresponding to the bleached color which in turn corresponds to the analyte chloropyrifos concentration was obtained by subtracting the absorbance of the blank solution from that of test solution.
Gas Chromatography
Instrumentaion: The analytical method was standardized by processing spiked samples in triplicate. Control samples were processed along with spiked ones.The details are given in table1.
Table 1: Instrumental parameters
| S.No | Parameters | Make | Specifications |
| 1. | Gas Chromatography | AGILENT-6820 | |
| 2. | Detector | ECD | |
| 3. | Carrier gas | Nitrogen | 99.99% |
| 4. | Solvent | Hexane | HPLC grade |
| 5. | Injection volume | Hamilton | 0.5 mic.L |
| 6. | column | Intercap | 30M length, 0.25 mmID,0.25 mic DF |
| 7. | Oven temperature programming | Endosulfan | 3 min 70.c,2-25 min 108.c |
Results and Discussion
The method is based on the alcoholic alkaline hydrolysis with potassium hydroxide.The reaction with potassium hydroxide in presence of an acid reagent forms an orange yellow colorand measured at 605nm.The absorption maximum is shown in figure1. The decrease tendency in absorbance is proportional to endosulfan.The Beer’s law is obeyed in the range of 0.5-11 µg/mL.
The consumption pattern of pesticides from agricultural source[12] is given in figure 2.
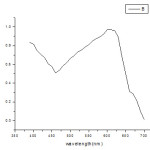 |
Figure 1: Absorption maxima of chloropyrifos |
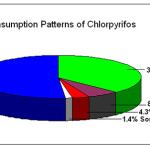 |
Figure 2: consumption pattern of pesticides
|
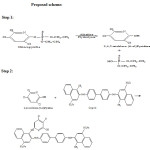 |
Scheme 1 |
Presence of pesticide residues in water,fruits and vegetables has become global phenomenon.For the development of solid phase extraction conditions , a volatile solvent system must be used, as rapid evaporation of a large volume would be required in sample preparation without causing loss of volatile pesticides. The solvent system must be sufficiently polar to extract most polar pesticides. A flow rate of 0.5 rnL/min was sufficient to recover all the pesticides except hexaconazole. It was noted that the solid phase extraction should not be left dry after conditioning. This could result in a significant loss of pesticides.It was observed that increasing the polarity of solvent gives lower recoveries. Twelve samples of Water, Fruits and Vegetables were collected from Agricultural fields in Sabbavaram,Visakhapatnam district for the determination of chloropyrifos are tabulated in table 2
Table 2:Chloropyrifos in water, fruits and vegetables in sabbavaram, Visakhapatnam district
| S.no |
Name of the item |
Pesticide residue,mg/Kg |
|
1. |
Water Banana field |
0.30,0.29,0.27 2.12,2.06,2.08 |
|
2. |
Fruits Pine-apple grapes |
2.86,2.71,2.39 5.62,5.12,5.09 |
|
3. |
Vegetables Carrot
Cabbage |
5.59,5.11,4.99 5.60,5.76,5.14 |
The present method was tailor-made in view of the previous information about the most prevalent pesticides in the area. From the results it can be seen that chloropyrifos was detected in the range of 0.27-5.62 mg/ kg.The recoveries for chloropyrifos in various samples ranged from 75-98% with coefficient of variance 1.3-7.6%.The various chromatograms for the determination of endosulfan were given in figures 3-8.
 |
Figure 3 |
 |
Figure 4
|
 |
Figure 5 |
 |
Figure 6 |
 |
Figure 7
|
 |
Figure 8 |
Conclusion
GC-ECD has great potential for determining organochlorine pesticides in food extracts at low levels. In general, chloropyrifos residues in food are well below the tolerance levels established for various food types by the FAO/WHO. The results from this study showed that recoveries obtained were comparable to those obtained from the established silica gel cleanup method, However lower recoveries occasionally occurred for chloropyrifos pesticide in water,fruits and vegetables using both cleanup methods. The benefits of the solid phase extraction method are less solvent consumption, no hazardous solvent is used, no cross-contamination and shorter analysis time.
Acknowledgement
The author thanks the head and faculty of department of Chemistry, G.I.T, GITAMUniversity for their support and encouragement.
References
- Muller, Franz, Agrochemicals: Composition, Production, Toxicology, Applications. TorontoWiley-VCH. Ed (2000), p. 541.
- Rauh, et al., Pediatrics, 118, 1845-1859,(2006)
- Whyatt, et al., Environmental Health Perspectives, 112, 1125-32,(2004)
- Thrasher, Ph.D., Jack D.; Gunnar Heuser, Alan Broughton, Archives of EnvironmentalHealth, , 57(2002),181-187.
- Hong dai, Environ Health Prev Med. 8(4), (2003),139–145.
- ElosaD. Caldas, Maria Hosana conceicao, Maria Clara C. Miranda, Luiz cesar K. R. deSouza and Joaq. , J.Agric. Food Chem. 49,4521-4525,(2001)
- JanghelE.K, .RaiJ.K. M.K.Rai, V.K.Gupta, J. Braz. Chem. Soc., 18, (2007).
- PatilV.B and ShingareM.S ., Analyst, 119,415-416.(1994)
- D.NThanh, Ji E.Y Dae, M. L., Gae H. L., Food Chemistry, 110 ,207-213.,(2008)
- Blasco.C,Fernadez,M J.of.Chrmatography, 77,1030.(2004)
- A.Bouaid .Ramos, J.of.Chrmatography, 13,939(2010).
- Source: http://www.speclab.com/compound.htm

This work is licensed under a Creative Commons Attribution 4.0 International License.









