A Comparative Study on the Phytoextraction of five Common Plants Against Chromium Toxicity
B. Dheeba and P. Sampathkumar
Department of Chemistry and Biosciences, Srinivasa Ramanujan Centre, SASTRA University, Kumbakonam - 612 001 (India)
Phytoextraction is a remediation technology that uses plants to remove heavy metals from soil. The success of a phytoextraction process depends on adequate plant yield (aerial parts) and high metal concentrations in plant shoots. A paper towel experiment was conducted to investigate the effects of plants sunflower (Helianthus annuus), maize (Zea maize), pearl millet ( Sorghum bicolour), green gram (Vigna radiata) and ground nut (Arachis hypogaea). Seeds of these plants were exposed to five different concentrations (10, 20,30,40,50 ppm ) of Cr as potassium dichromate. The ecotoxicological effects of hexavalent Chromium (Cr) on germination, early seedling growth and chlorophyll content of five plants were investigated. Cr accumulating capacity of those plants was compared. Different species showed different levels of tolerance to Cr pollution. Total chlorophyll content declined progressively with increasing concentrations of heavy metals. The maximum chromium accumulation capacity of roots was in the order of Zea mays > Sorghum bicolour > Helianthus annus > Arachis hypogaea > Vigna radiata and the amount is 5.56 , 3.65, 3.56, 0.8 and 0.08 ppm respectively.
KEYWORDS:Phytoremediation; Hexavalent chromium; Root and shoot length; Chlorophyll
Download this article as:| Copy the following to cite this article: Dheeba B, Sampathkumar P. A Comparative Study on the Phytoextraction of five Common Plants Against Chromium Toxicity. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Dheeba B, Sampathkumar P. A Comparative Study on the Phytoextraction of five Common Plants Against Chromium Toxicity. Available from: http://www.orientjchem.org/?p=23456 |
Introduction
In recent decades there has been increasing concern with heavy metal contamination, because of their toxicity to microorganisms, plants and animals. Unlike most organic contaminants are non-biodegradable and can accumulate in living tissues ( Srivastava et al., 2003). A variety of anthropogenic sources, such as mining processing, electroplating, wood preservation, iron and steel production, pigment manufacture, smelters, power station industry, production and application of metal-containing pesticides, can lead to soils acquiring heavy metal contents substantially in excess of natural levels. Some of these metals, at relatively low concentrations, may stimulate the biological life (Yamaji et al., 2001; He et al., 2005), while increased concentrations in the environment can be detrimental to a variety of living species ( Chatterjee and Chatterjee, 2000; Naimo, 1995). The toxic potential of heavy metals in soil depends on soil composition, particularly on amount and type of clay minerals, organic matter and iron and manganese oxides (Covelo et al., 2007). All these properties influence metal mobility and availability, and therefore, influence their release and their interaction with other components of the ecosystem, such as plants. Chromium is an important metal that is usually encountered in the environment at oxidation states of (+III) and (+VI) ( Kotas and Stasika, 2000). Each of these oxidation states has very different biological and toxicological properties ( Kimbrough et al., 1999). Hexavalent chromium has a high solubility, being a well-established carcinogen based upon animal, human, and in vitro assessment data( Costa,1997; Gheju, 2005). On the contrary, the reduced form of chromium, Cr(III), is much less toxic and usually precipitates as hydroxides ( Rai et al., 1987)
Increased world population has resulted in the pollution of the environment. The main factors responsible for pollution and other type of environmental degradation in any community caused by combined effects of effluents and due to modern technology (Meadows et al., 1992) Chromium enters the food chain through consumption of plant material. A high concentration of Cr has been found to be harmful to vegetation. As the chromium concentration in plants increases, it adversely affects several biological parameters. Ultimately there is loss of vegetation, and land some times becomes barren ( Dube et al., 2003). In recent years, contamination of the environment by chromium has become a major concern. Chromium is used on a large-scale in many different industries, including metallurgy, electroplating, production of paints and pigments, tanning, wood preservation, chemical production, and pulp and paper production ( Zayed and terry, 20030). These industries have be-come especially large contributors of Cr pollution, which can ultimately have significant adverse biological and ecological effects. Symptoms of Cr phytotoxicity include inhibition of seed germination or of early seedling development, reduction of root growth, leaf chlorosis and depressed biomass ( Sharma et al., 1995). There are many studies on Cr toxicity in crop plants. Chromium significantly affects the metabolism of plants such as barley Ali et al.,2004), citrullus (Dube et al.,2003) , cauliflower (Chatterjee and Chaterjee, 2000) ,vegetable crops ( Zayed et al., 1998) , wheat ( Sharma et al., 1995) and maize (Sharma and Sharma, 1994).
Chromium is highly toxic non-essential element for microorganism and plants (Cervantes et al., 2001). The source of chromium in environment are both natural and anthropogenic, natural source include burning of oil and coal, petroleum from ferro chromate refractory material, chromium steels, pigments oxidants, catalyst and fertilizers. This element is also used in metal plating tanneries and oil well drilling ( Abbassi et al., 1998) . Sewage and fertilizers are also the sources of chromium ( Pillay et al., 2003). Chromium has its effect on certain enzymes such as catalase, peroxidase, a cytochrome oxidase, which have iron as constituent. Agarwala and Kumar (1962) has reported that stimulation of catalase activity at excess supply of chromium occurred. Marked toxicity of chromium was found with respect to photosynthetic pigment, photosynthesis, nitrate reductase activity and protein content of some alga (Rai et al., 1992). The direct interaction of metal with cellular components can initiate variety of metabolic responses final y leading to a shift in the development of the plant (Assche Van and Clijsters,1990). Chromium toxicity produces chlorosis and necrosis in plants (Cervantes et al.,2001) . Several polluting metal and compounds are discharged into the water streams by tanneries.
Plant species have different responses to heavy metal pollution of soils. Although it may exist a relationship between heavy metal accumulation and plants tolerance, many plant species grow on contaminated soils and yet do not accumulate metals (Gabberielli et al., 1990). Plants that possess the ability to tolerate, uptake and accumulate high levels of metals in their biomass are termed as hyperaccumulators (Brown et al., 1995). The technique in which plants are used for the removal of heavy metals is known as phytoremediation. With these aspects, the present investigation was made to study the effect of different concentrations of chromium on the growth, chlorophyll content and phytoremediation property of Zea mays, Helianthus annus, Sorghum bicolour, Vigna radiata and Arachis hypogaea.
Materials and Methods
Test Plants
The seeds of Zea mays, Helianthus annus, Sorghum bicolour, Vigna radiata and Arachis hypogaea were collected from local agricultural centre and authenticated by Dr. S.Kalavathy, Associate professor of Botany, Bishop Heber college, Trichy. Seeds were surface sterilized in 0.5 % sodium hypochlorite solution for 20 minutes and washed thoroughly with distilled water.
Experimental setup
Potassium dichromate solution was used as a source of Cr(VI). The experimental design was completely randomized with 6 replications The treatments were randomized in order to eliminate experimental bias. Five Cr levels ranging from 10,20,30,40 and 50 ppm including distilled water (control) were selected. Applications of chromium up to 50ppm had nominal effect, while there was no plant survival beyond 50 ppm therefore Cr levels selected for this study were 0(control)10,20,30, 40 and 50ppm. The paper towels were made by using filter papers in the size of 15X15 cm and wet with distilled water. Surface sterilized seeds (6) were placed in the middle of the paper and rolled by having a plastic sheet in same size for support. Then rolled towel is kept in 20ml of different concentrations of Cr in a cup and seedling were allowed to grow for 12days, after which they were removed from paper towel. The physical parameters such as root length, shoot length and fresh weight were analysed by using appropriate methods.
Plant Analysis
Chlorophyll (a, b, and total) analysis
Pigment contents of 12 days old plants were extracted by using the formula of Arnon, 1949. The leaves were chopped into small pieces that were extracted with 80%acetone. The absorbance was measured at 645 nm and 663 nm for chl a,b and total chl respectively by using spectrophotometer (Hitachi Model-U 2001 Japan).Then chlorophyll a, b & total chlorophyll were calculated. ( Lichtenthaler and Wellburn, 1983)
Chl a (mg g-1 leaf fresh weight) = [12.7(OD663) -2.69 (OD645)] × V/1000 × W.
Chl b (mg g-1 leaf fresh weight) = [22.9(OD645) -4.68 (OD663)] × V/1000 × W.
Total Chl (mg g-1 leaf fresh weight) = [20.2(OD645)-8.02(OD663)] ×V/1000 × W.
Where OD = Optical Density. V = Volume of sample. W = Weight of sample.
Extraction of metal from plant samples
The harvested plant was separated out as root and shoot, crushed into powder and incinerated at high temperature. The ash obtained from the incinerated plant samples were treated with concentrated HCl and the metals analyzed using Atomic Absorption Spectrometer (AAS) (APHA, 1990).
Results and Discussion
Physical parameters
Shoot length
Under various experimental conditions, the shoot lengths of Zea mays, Helianthus annus, Sorghum bicolour, Vigna radiata and Arachis hypogaea were analysed. The shoot lengths of three plant species (Helianthus annus, Vigna radiate and Arachis hypogaea ) were reduced by more than 50% when compared to control (without Cr ). In Vigna radiata and Arachis hypogaea plants, root and shoot growth were similarly sensitive. For example, the shoot lengths of all plants are represented in the following graph which supports significant alteration. In particular it is reduced more than 60% when it is compared the other treatments with controlled treatment. ( Fig1 ).
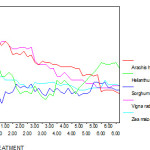 |
Figure 1: The shoot length ( cm) of experimental plants under various experimental conditions
|
Further, the – chart, R-chart for shoot length in Arachis hypogaea under 6 treatments are obtained. The – chart shows that the shoot lengths are not control but the R-chart indicated that the ranges are with in the control limits. (Fig 2 a and b)
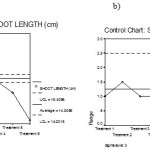 |
Figure 2: Mean chart (a) and Range chart (b) of shoot length of experimental plants under various experimental conditions.
|
Moreover, the ONE WAY ANOVA for Shoot length (cm) is obtained which is shown in the Table 1. The calculated F value is 427.692. But the tabulated F value at (5, 30) degrees of freedom at 1% and 5% level of significance is 3.70 and 2.53 respectively. Hence calculated F Value > tabulated F value at both level of significances. Therefore, the difference between the shoot lengths is highly significant. Also the Karl Pearson’s correlation coefficient and Spearman’s rank correlation coefficients between the treatments and shoot lengths are – 0.973 and – 0.987 respectively. Hence they are negatively correlated and correlation is significant at the 0.01 level (2-tailed).
Table 1: ONE WAY ANOVA for Shoot length (cm) of experimental plants under various experimental conditions.
| Sum of Squares | Degrees of freedom | Mean Square | F | |
| Between Groups | 540.556 | 5 | 108.111 | 427.692 |
| Within Groups | 7.583 | 30 | .253 | |
| Total | 548.139 | 35 |
Root length
The root lengths of Zea mays, Helianthus annus, Sorghum bicolour, Vigna radiata and Arachis hypogaea in different experimental conditions decreased when Cr concentration is increased. In Arachis hypogaea the root length is reduced to 66% and shoot length is also markedly reduced to 60 % at 50 ppm Cr concentration when compared to control plants. Among five plants Zea mays is more tolerant to Cr toxicity followed by Sorghum bicolor in which only upto 13% reduction in root and shoot length was noticed in 50 ppm Cr concentration when compared to control. For example, the root lengths of Arachis hypogaea are represented in the following graph which shows that the shoot lengths are decreased gradually with respect to the treatments. In particular it is reduced more than 60% when it is compared the other treatments with controlled treatment. (Fig 3 )
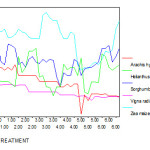 |
Figure 3: The root length( cm) of experimental plants under various experimental conditions
|
Further, the – chart, R-chart for root length in Arachis hypogaea under 6 treatments are obtained. The – chart shows that the root lengths are not control but the R-chart indicated that the ranges are with in the control limits. ( Fig 4 a and b)
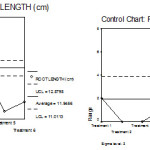 |
Figure 4: Mean chart (a) and Range chart (b) of root length of experimental plants under various experimental conditions.
|
The ONE WAY ANOVA for root length (cm) is obtained which is shown in the Table 2. The calculated F value is 63.507. But the tabulated F value at (5, 30) degrees of freedom at 1% and 5% level of significance is 3.70 and 2.53 respectively. Hence calculated F Value > tabulated F value at both level of significances. Therefore, the difference between the root lengths is highly significant. The Karl Pearson’s correlation coefficient and Spearman’s rank correlation coefficients between the treatments and root lengths are also – 0.922 and – 0.967 respectively. Hence they are negatively correlated and correlation is significant at the 0.01 level (2-tailed). But, the Karl Pearson’s correlation coefficient and Spearman’s rank correlation coefficients between the shoot lengths and root lengths are 0.844 and 0.943 respectively. Hence they are positively correlated and correlation is significant at the 0.01 level (2-tailed).
Table 2: ONE WAY ANOVA for root length (cm) of experimental plants under various experimental conditions.
| Sum of Squares | Degrees of freedom | Mean Square | F | |
| Between Groups | 528.104 | 5 | 105.621 | 63.507 |
| Within Groups | 49.894 | 30 | 1.663 | |
| Total | 577.998 | 35 |
The higher concentration of effluent having heavy metals decrease enzyme dehydrogenase activity that is considered as one of the biochemical change which may have disrupt germination and seedling growth ( Murkumar and Chauan, 1987). Studies on heavy metal tolerance in plants indicate that root growth is particularly sensitive to heavy metals ( Punz and Sieghardt, 1993). Copper toxicity affected the growth of Alyssum montanum ( Ouzounidou, 1994) and Brassica juncea ( Singh and Tiwari, 2003). Reduction in root growth due to heavy metals has also been reported in wheat seedlings ( Oncel et al., 2000). The number of leaves and branches, root and shoot length and biomass decreased as concentration of Cr increased in egg plant and tomato (Purohit et al., 2003) and in barley ( Arey and Rana, 2003). Reduced growth in terms of root and shoot lengths at increasing doses of chromium might be due to adverse effect of this metal on auxin synthesis in plants more during early stages of their growth. Increasing concentrations of chromium caused significant reduction in root length and shoot length.
Fresh Weight
Fresh weight of plants are not much affected at 10 and 20 ppm Cr concentration but affected at higher concentration of Cr in all five species of plants. When compared to control plants at 50ppm, fresh weight of Vigna radiata and Arachis hypogaea plants were reduced to 50%. In all plants at 5ppm concentration, fresh weight is increased. Presence of Zn at higher concentrations retarded the growth and development of plants by interfering with certain important metabolic processes ( Alia., 1995) . The following are the sample for the analysis in Arachis hypogaea. ( fig 5 ).
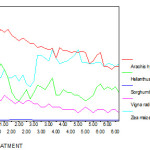 |
Figure 5: The fresh weight (g) of experimental plants under various experimental conditions
|
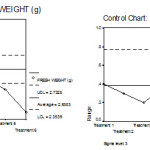 |
Figure 6: Mean chart (a) and Range chart (b) of fresh weight of experimental plants under various experimental conditions.
|
Moreover, the calculated F value is 48.151. But the tabulated F value at (5, 30) degrees of freedom at 1% and 5% level of significance is 3.70 and 2.53 respectively. Hence calculated F Value > tabulated F value at both level of significances. Therefore, the difference between the fresh weights is highly significant. (Table 3)
Table 3: ONE WAY ANOVA for fresh weight (g) of experimental plants under various experimental conditions.
| Sum of Squares | df | Mean Square | F | |
| Between Groups | 5.279 | 5 | 1.056 | 48.151 |
| Within Groups | .658 | 30 | .022 | |
| Total | 5.937 | 35 |
Plant pigments
Increasing concentrations of chromium caused significant reduction in Chl a, b and total chl. In all five variety of plants pigment levels significantly decreased at 10, 20, 30, 40 and 50 ppm of chromium as compared to control. In Zea mays, Sorghum bicolour and Helianthus annus plants Chl a is reduced at higher concentration of Cr than Chl b. In Vigna radiata and Arachis hypogaea group both Chl a and Chl b are reduced more than 50% at higher concentration of chromium treatment when compared to control group plants.(Fig 8 -12).
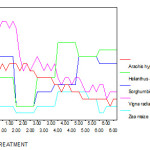 |
Figure 7 :The chlorophyll a ( mg/g) of experimental plants under various experimental conditions
|
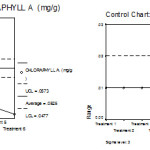 |
Figure 8: Mean chart (a) and Range chart (b) of chlorophyll a of experimental plants under various experimental conditions.
|
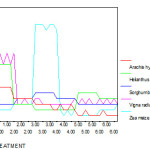 |
Figure 9 :The chlorophyll b ( mg/g) of experimental plants under various experimental conditions
|
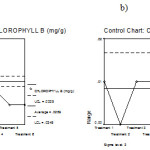 |
Figure 10: Mean chart (a) and Range chart (b) of chlorophyll b of experimental plants under various experimental conditions.
|
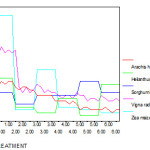 |
Figure 11: The total chlorophyll ( mg/g) of experimental plants under various experimental conditions
|
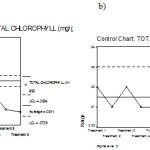 |
Figure 12: Mean chart (a) and Range chart (b) of total chlorophyll of experimental plants under various experimental conditions.
|
Photosynthesis was reduced significantly by increasing Cr(VI) concentrations in the growth medium. The modification of total chlorophyll content and the chl a/b ratio is noted under toxic conditions. Chl a and b content decreased significantly in the 50 ppm, showing the negative effect of Cr(VI) on processes of chlorophyll biosynthesis. The increase in chl a and b content at the 10ppm Cr(VI) dose was noticed and that suggested that low concentrations of Cr(VI) increases Chl synthesis in all five plant species. The decrease in chlorophyll content with increasing Cr(VI) dose has been described in the literature repeatedly (Liu et al., 2008) It is thought to be related to direct inhibition of chlorophyll pigment synthesis. Other authors have reported a decrease in chlorophyll content correlated with metal concentration (Panda and Choudhury, 2005; Scoccianti et al., 2006). Chromium possesses the capacity to degrade δ-aminolevulinic acid dehydratase, an important enzyme involved in chlorophyll biosynthesis, thereby affecting δ-aminolevulinic acid (ALA) utilization; this results in the buildup of ALA and reduction of the level of chlorophyll ( Vajpayee et al., 2000) . Chromium, mostly in its hexavalent form, can replace Mg ions from the active sites of many enzymes. Cr(VI) also causes Fe deficiency in stressed plants, disrupting chlorophyll biosynthesis ( Liu et al., 2008). The chl a/b ratio decreased when the plants were exposed to 40 μM and 100 μM Cr(VI), perhaps due to faster breakdown or decreased synthesis of chl a as compared to chl b, although chl b also decreased ( Vajpayee et al., 2000)
The decrease in the concentrations of chlorophyll a and b and total chlorophyll in plants exposed to excess supply of Cr is similar to the effects of other pollutant elements (Lee et al., 1993).The decrease in chlorophyll concentration may be the result of an inhibited photosynthetic electron transport ( Bohner et al., 1980) and decomposition of the chloroplast membrane with excess Cu ( Sandmann and Boger,1980). The adverse effects of heavy metals in excess in plants may be because these metals interfere in the formation of chlorophyll either through the direct inhibition of an enzymatic step or through the induced Fe deficiency (Van Assche and Clijsters, 1990). Heavy metal induced reduction in total chlorophyll has been reported in pigeon pea due to Cd and Ni stress ( Shooran et al., 1990) and in Brassica juncea due to Cd stress ( Singh and Tiwari,2003). Reduction in chlorophyll content due to its degradation and unfavourable effect on photosynthetic electron transport has been reported due to copper toxicity in sensitive species of Silene compecta and Thalpsi ochrolucum ( Ouzouidou,1994).
Chromium accumulation in plants
The maximum chromium concentration in shoot was found in 50 ppm chromium treated Zea mays plants i.e 2.86 ppm and the lowest amount is in the shoot of Vigna radiata plants (0.061ppm) in 50ppm concentration.
In all five varieties of plants the accumulation of chromium is higher in roots than shoots this is because of poor translocation from root to shoot. While comparing five group of plants Zea mays, Sorghum bicolour and Helianthus annus plants uptake more chromium than other two species. The maximum chromium accumulation capacity of roots was in the order of Zea mays > Sorghum bicolour > Helianthus annus > Arachis hypogaea> Vigna radiata and the amount is 5.56 , 3.65, 3.56, 0.8 and 0.08 ppm respectively. (Fig 13 – 16)
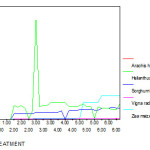 |
Figure 13: Shoot Chromium (mg/g) content of experimental plants under various experimental conditions
|
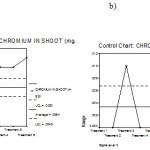 |
Figure 14: Mean chart (a) and Range chart (b) of shoot chromium content of experimental plants under various experimental conditions.
|
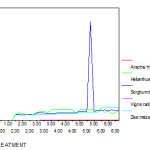 |
Figure 15: Root Chromium (mg/g) content of experimental plants under various experimental conditions
|
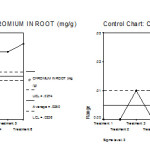 |
Figure 16: Mean chart (a) and Range chart (b) of root chromium content of experimental plants under various experimental conditions.
|
Chromium produced a significant reduction in the growth of P. juliflora. The effect was mainly observed on root and shoot and to a lesser extent on seedling length. The inhibition of growth by chromium could be mainly due to the accumulation of chromium in the roots. Bishoni et al., ( 1993) observed that hexavalent chromium applied as potassium di chromate did not affect the percentage germination of pea seed but the growth of radicles and plumule were significantly suppressed above 20 ppm concentration and the deleterious effect of chromium was more pronounced on the growth of roots than on shoots. The present study showed the deleterious effect of chromium on roots due to its accumulation in the roots which was extremely limited in the shoots .Accumulation of chromium in roots was 100 fold higher than that by shoot regardless of the chromium supplied. Low concentration of chromium was stimulatory to the plant growth while other workers contradicted it and observed that chromium is inhibitory and toxic to plant growth. Chromium toxicity manifested itself in plants by inhibiting the growth more or less, showing chlorosis with small brownish-red or purple leaves and necrotic lesions. High chromium concentration inhibits photosynthesis and greatly inhibits the root growth. It is therefore evident that chromium affects the plant growth mainly by damaging the root while its translocation into other parts of the plant is of minor importance.
Deleterious effect of chromium on plants suggests that chromium is very much toxic in nature and considered inhibitory to plant ( Sharma and Arey, 2000). It is concluded that among the five plants studied Zea mays and Sorghum bicolour shown better chromium accumulation when compare with other plants with reference to growth. Hence these plants can be suggested for phytoremediation with further studies.
References
- Abbassi SS, Abbassi N, Soni R. Heavy metals in the environment, Mittal Publication, New Delhi, India. 1998.
- Aery C, Rana DK. Growth and cadmium uptake in barley under the cadmium stress. Journal of Environ Biology. 2003; 24: 117-123.
- Agarwala SC, Kumar A. The effect of heavy metals and bicarbonate excess on sun flower plants grown in sand culture with special reference to catalase peroxidase. J. Ind. Bot. Soc., 1962; 41: 72-77.
- Ali NA, Ater , Sunahara, Robidoux PY. Phytotoxicity and bioacuumulation of copper and chromium using barley (Hordeum Vulgare L.) in spiked artificial and natural forest soils. Ecotoxicology and environmental safety.2004; 57: 363-374.
- Alia KVSK, Prasad SK,. Pardha Saradhi P. Effect of zinc on free radicals and praline in Brassica juncea and Ca janus caj anas. Phytochemistry. 1995; 39: 45-47.
- APHA Standard methods for the examination of waste and waste water (14 th Edition) American Public Health Association, New York;1990.
- Arnon DT. Copper enzymes in isolated Chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949; 24, 1-15.
- Assche Van F, ClijstersH. Effects of metals on enzyme activity in plants. Plant Cell Environ., 1990;13: 195-206 .
- Bishoni NR, Dua A, Gupta VK, Sawhney SK. Effects of chromium on seed germination, seedling growth and yield of peas. Agriculture Ecosystem .and Environment. 1993; 47: 47-57.
- Bohner H, Bohme H, Boger P. Reciprocal formation of cytochrome C-553 and the infuence of cupric ions on photo- synthetic electron transport. Biochimica et Biophysica Acta 1980; 592: 103 – 12.
- Brown SL, Chaney RL, Angle JS, Baker AJM. Soil Science Society of America Journal. 1995; 59, 125.
- Cervantes C, Campos-Garcia J, Debars S, Gutierrez-Corona F, Loza-Tavera H. Carlos-Tarres-Guzman, Moreno-Sanchez R . Interaction of chromium with Microgenesis and plants. FEMS Microbiol. Rev. 2001; 25: 335-347.
- Chatterjee J, Chatterjee C. Phytoxicity of cobalt, chromium and copper in Cauliflower. Environ. Pollut. 2000; 109: 69-74.
- Costa M . Critical Reviews in Environmental Science and Technology. 1997; 27: 431
- Covelo EF, Vega FA, Andrade ML. Journal of Hazardous Materials. 2007; 140: 308.
- Dube BK, Tewari K, Chatterjee J, Chaterejee C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere. 2003; 53: 1147-1153.
- Gabbrielli R, Pandolfini T, Vergnano O, Palandri MR. Plant and Soil. 122, (1990) 271.
- Gheju M. Chromium and the environment. Politehnica” Publishing House, Timisoara, 2005.
- He ZL, Yang XE, Stoffella PJ. Journal of Trace Elements in Medicine and Biology 2005; 19, 125.
- Kimbrough DE, Cohen Y, Winer AM. Critical Reviews in Environmental Science and Technology 1999; 29: 1.
- Kotas J, StasickaZ. Environmental Pollution. 2000; 107: 263.
- Lee CV, Pak CH, Choi JW, Self JR. Induced micro-nutrients toxicity in Petunia hybrida. Journal of Plant Nutrition .1993; 15, 32: 339.
- Lichtenthaler HK,. Wellburn AR. Determination of total carotenoids and chlorophylls a, b in leaf extracts in different solvents. Biochem.soc.Trans.1983;11: 591-592.
- Liu DH, Zou JH, Wang M, Jiang WS. Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresource Technology. 2008; 99: 2628–2636.
- Meadows DH, Meadows DL, Randers. Beyond the limits Earthscan publication, London. 1992.
- Mur Kumar CV, Chauan PD. Influence of water pollution on germination of gram (Cicer arietinum L.)In: Current pollution Research in India. (Eds: Triveni pk,. Goel PK). 1987.
- Naimo TJ. Ecotoxicology 1995; 4: 341.
- Oncel I, Kele Y, Ustun AS. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environmental Pollution. 2000; 10: 315-320.
- Ouzounidou G . Copper induced changes on growth, metal content and photosynthetic functions of Alyssum montanum L.plants. Environmental Experimental Botany. 1994; 34: 165-172.
- Panda SK, Choudhury S. Chromium stress in plants. Brazilian Journal of Plant Physiology. 2005; 17: 95–102.
- Pillay AE, Williams JR, EL Mardi MO, AI-Lawati SMH, AI-Hadabbi MH, Hamdi AAI.Risk assessment of chromium and arsenic in date palm leaves used as livestock feed. Environ. Intl., 2003;1048: 1-5
- Punz WF, Sieghardt H. The responses of root of herbaceous plants species to heavy metals. Environmental Experimental Botany. 1993; 33: 85-98.
- Purohith S, Varghese TM, Kumari M. Effect of Cr on morphological features of tomato and brinjal. Indian Journal of Environmental Biology. 2003; 8: 17-22.
- Rai D, Sass BM, Moore DA. . 1987;26: 345.
- Rai UN, Tripathi RD, Kumar N. Bioaccumulation of chromium and toxicity on growth, photosynthetic pigments, photosynthesis, in vivo nitrate reductase activity and protein content in chlorococcalear green alga, Glaucocystis nostochinearum ltzigsohn. Chromosphere. 1992;25: 721-732.
- Sandmann G, Boger P. Copper deficiency and toxicity in Scenedesmus. Zeitschrift fur Planzenphysiologie. 1980; 98:(1980) 53-59.
- Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A. Uptake and toxicity of Cr(III) in celery seedlings. Chemosphere. 2006; 64: 1695–1703.
- Sharma A, Aery NC. Studies on the phytoremidiation of zinc tailings. I. Growth performance of wheat. Vasundhara. 2000;6: 45-50.
- Sharma DC, Chatterjee C, Sharma CP. Chromium accumulation by barley seedlings (Hordeum vulgare L,). Journal of experimental botany. 1995; 25: 241-251.
- Sharma DC, Sharma RC. Pant Chromium uptake its effects on certain plant nutrients in maize (Zea mays L. CV Ganga 5). Journal of environmental science and health, Part A .1994; 29: 941-948
- Sheoran IS, Single HR, Singh R.Effect of Cd and Ni on photosynthesis and the enzymes of photosynthetic carbon reduction cycle in pigeon pea (Cajanus cajan). Photosynthesis Res., 1990; 23: 45-35.
- Shrivastava R, Upreti RK, Chaturvedi UC. FEMS Immunology and Medical Microbiology 2003 ; 38, 65.
- Singh PK, Tewari SK. Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L.plants. Journal of Environmental Biology.2003; 24:107-117.
- Vajpayee P, Tripati RD, Rai UN, Ali MB, Singh SN. Chromium (VI) accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content in Nymphaea alba L. Chemosphere 2000; 41: 1075–1082.
- Yamaji S, Tennant J, Tandy S, Williams M, . Srai SKS, Sharp P. FEBS Letters 2001; 507,137.
- Zayed A, Lytle CM, Qian JH, Terry N. Chromium accumulation, translocation and chemical speciation in vegetable crops, Planta. 1998; 206: 293-299.
- Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant and soil. 2003; 249:139-156.

This work is licensed under a Creative Commons Attribution 4.0 International License.









