Waste Tendu Leaves From Beedi Industry Utilized For Removal of Sulphur Dye From Textile Waste Water
S. A. Paul* and S. K. Chavan
P.G. Department of Chemistry, D.B.F. Dayanand College of Arts and Science, Solapur-413002, Maharashtra (India).
An activated carbon as adsorbent is derived from waste tendu leaves (Diospyros melanoxylon) from local Beedi (Cigarette) industry for removal of sulphur dye from textile waste water. Tendue leaves were chemically treated with sulphuric acid and activated carbon prepared was analyzed by pH, moisture content, ash content, bulk density and BET surface area. The adsorption studies were performed as a function of different concentration of dye solution, contact time, pH and different adsorbent doses. The kinetics of the adsorption process follows the pseudo second-order kinetic model. Equilibrium data was analyzed using Langmuir, Freundlich and Tempkin isotherm models. The suitability of the data in different model of isotherm is determined from correlation coefficient (R2) value. The results of all isotherms model shows correlation coefficient (R2) value close to 1 and hence results fit into adsorption data. The results showed that tendu leaves a solid waste disposal from Beedi industry can be used as effective biosorbent for removal of sulphur dye from textile waste water.
KEYWORDS:Tendue leaves; adsorption; activated carbon; isotherm
Download this article as:| Copy the following to cite this article: Paul S. A, Chavan S. K. Waste Tendu Leaves From Beedi Industry Utilized For Removal of Sulphur Dye From Textile Waste Water. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Paul S. A, Chavan S. K. Waste Tendu Leaves From Beedi Industry Utilized For Removal of Sulphur Dye From Textile Waste Water. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23986 |
Introduction
Ecological balance is threaned by increasing environmental pollution due to rapid industrialization in the world and textile industries are one of the sources of the environmental pollution1. The textile industries not only causes water pollution by discharging large amount of waste water into various receiving bodies but also air pollution by ejecting different gases into atmosphere2. In fact, major pollution due to textile industries are discharging colored effluents containing various types of dyes and most of the dyes used are stable towards photo-degradation and biodegradation due to their complex structure3 and it is disastrous for the environmental beings. Many of the organic and inorganic dyes are hazardous and may affect aquatic life and the food chain and sulphur dyes are not exception for this. In addition some dyes or their metabolites are toxic, mutagenic or carcinogenic in nature4. Thus colored effluents discharged by textile industry are dangerous source of environmental contamination not only from aesthetic point of view but also due to its toxicity to aquatic life.5
So it is essential to remove dye from colored effluent for safe discharge in receiving water bodies and prevent water pollution due to textile effluent. Various methods are available for removal of dye like coagulation using alum, flocculation using polyelectrolyte, ozonation, chemical, biological, membrane and adsorption by activated granular carbon but these have significant disadvantage such as high cost, high energy requirement etc. therefore these methods are not suitable for developing country like India. Biosorption using reusable industrial waste offer a reliable alternative to these traditional methods for dye removal in economically and eco-friendly.
Adsorbents prepared from agriculture waste and by product have been widely studied. A number of low cost adsorbents, such as activated carbon prepared from various wastes6-8, baggas fly ash5, Rice Husk9-13, coal fly ash14, Tendue leaves3, 15. Trpioca peel activated carbon (TPAC) 16, Granulated carbon, powdered carbon, Aluminum oxide17, Red ochre18 and Datura Stramonium7 have been studied for adsorption of dye.
In the present study Tendue leaves (Diospyros melanoxylon) are used as adsorbents for removal of sulphur dye from textile waste water. Tendue leaves are important material used in (hand rolling) Beedi industries in India. About 7.5 million peoples are engaged in collection of tendu leaves and another 3 million are in beedi preparation by hand rolling process. Estimated revenues from tendu leaves are US$ 200 million per year.4 Around 300,000 tons of tendu leaf are produced annually across the India, from which 20% waste is remaining during manufacturing of beedi20. The purpose of this proposed work is to investigate the adsorption capacity of chemically activated tendu leaves. The effects of various parameters such as agitation time, adsorbent doses, concentration of dye solution and pH have been studied.
Material and Methods
Collection and preparation of biosorbent: Tendu leaves are used as beedi wrapper, beedi is smaller and less expensive than a cigarette and it is poor man’s cigarette in India. Small cutting pieces of tendu leaves were remaining during manufacturing of beedi, which itself causes environmental pollution. These waste tendu leaves are reused as adsorbent material for dye removal in the present study. Waste tendu leaves are collected from dumping site of Beedi industries in Solapur city, India. These leaves are cut into small pieces and washed with distilled and then dried at 70oC for 8 hours. The completely dried material was grinded into powder and again stored into air tight oven at room temperature.
Activated carbon from tendu leaves are prepared by treating the material with concentrated sulphuric acid in the ratio of 3:1 proportion and kept in air tight oven at 150oC-180oC for 48 hours. The black colored mass was washed with distilled water until it becomes free from excess of acid and further dried in oven at 105oC for 12 hours. Dried product is called as activated carbon tendu leaf reuse (ACTLR).
Preparation of Dye solution: A stock solution of sulphur black dye was prepared by dissolving 1gm of sulphur dye in 1000ml distilled water to make the concentration of stock solution is 100mg/l. To prepare desired concentration of dye solutions, take 10, 20, 40 and 50mg/l of stock dye solution and diluted to 100ml. For absorbance measurements study before and after treatment of ACTLR a UV-VIS spectrophotometer (SAMAZU, 1202D) IS USED. All absorbance measurements was done at λmax= 620 nm. Concentrations of dye before and after treatment of ACTLR are determined by standard calibration curve.
Adsorption Studies: The batch adsorption was carried out in 250 ml Borosil conical flask by mixing 1gm CATLR and 100 ml aqueous solution of dye at different concentration of (10, 20, 40, and 50). The conical flasks were kept on a magnetic stirrer and agitated for different time interval. The solution was filtered and filtrate was used for measurement of absorbance. Concentration of dye remaining after treatment is determined by standard curve. The adsorption capacity, qe were calculated from difference between the initial concentration and equilibrium concentration, which can be calculated as
qe = (Co-Ce) V X 1000/ M
qe = adsorption capacity in mg/g, Co and Ct are initial concentration and equilibrium concentration in mg/l, M= adsorbent in gm and V= volume of solution.
The adsorption behaviours of samples were studied by evaluating % removal efficiency of CATLR by relation,
% Removal = Co – Ce / Co x 100
Results and Discussions
Characteristics of adsorbent: The physical characteristics of chemically activated tendu leaves prepared are given in table no. 1. Analysis of CATLR are determined by standard methods 21, 22
Table 1: Characteristics of CATLR
|
Parameters |
Characteristics value |
|
pH |
8.06 |
|
Bulk density( g/ml) |
0.5984 g/ml |
|
Moisture content (%) |
3.88 % |
|
Ash content (%) |
4.47 % |
|
BET Surface Area m2/g |
270 m2/g |
The adsorption of sulphur dye in aqueous solution on activated tendu leaves were studied with the help of physico-chemical parameters such as contact time, pH and amount of absorbent, adsorbate and effect of agitation gradually.
Effect of contact time and Agitation time
The experiment was carried out with different concentrations 10, 20, 40, 50 mg/l of sulphur dye (100ml) and agitated with 1 gm of activated carbon tendu leaves at room temperature .Figure 2 shows the effect of initial concentration of dye and contact time on rate of adsorption. The adsorption at different dye concentration was rapid at initial stages and then decreases with the progress of adsorption. The uptake of sulphur dye on activated carbon nearly reaches equilibrium within four hours. These results are expected because our large numbers of surface active sites are available for adsorption at initial stage and after laps of time, the remaining surface sites are difficult to occupy, because of repulsion between solute molecules of the solid and bulk phase.
Effect of pH
The effect of pH and contact time on adsorption of sulphur dye is shown in figure no.3. It is evident from figure that maximum adsorption of sulphur dye at pH =8.06. The pH of solution is an important parameter for controlling the adsorption process. The nitration between adsorbate and adsorbent is affected by pH of an aqueous medium.
Effect of Adsorbent Dosage
A series of adsorption experiment was carried out with different adsorbent dosages at initial concentration of 20mg/l with constant volume of dye solution (100ml). Figure-4 shows the effect of adsorbent on adsorption process, as increased the dosages of adsorbent the amount of adsorption is also increases. This is due to availability of large number of active sites on adsorbent surface at higher dosages.
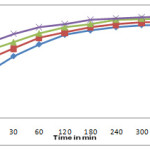 |
Figure 1:Treatment of Tendue leaves without stirring |
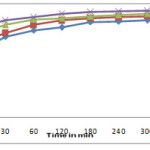 |
Figure 2: Effect of stirring treatment of Tendue leaves |
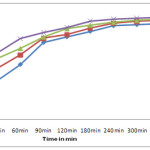 |
Figure 3: Effect of Adsorbent dosages |
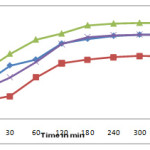 |
Figure 4: Effect of pH |
Kinetic model
Various adsorption kinetic models have been used to describe the adsorption of dyes. The pseudo first order rate equation23 and pseudo second-order model24, 25 have been widely used. From results it is shows that present adsorption process follows pseudo second-order kinetic model, because correlation coefficient shows greater i. e. 0.999 value. Pseudo second-order equation is based on the sorption capacity on the solid phase.
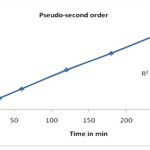 |
Figure 5: Pseudo second-order plot |
Adsorption isotherms: The analysis of isotherm data by fitting them into different model. Here, Langmuir, Freundlich and Tempkin model were used to describe the data derived from the adsorption of sulphur dye by CATLR. The applicability of three isotherms was compared by evaluating the correlation coefficients (R2) values.
Langmuir isotherm
In order to facilate the estimation of the adsorption capacities at various conditions, the Langmuir adsorption isotherm was applied24. The linearised Langmuir model can be given as,
1/qe = 1/KL + 1/KL1/Ce
Where Ce is concentration of dye in mg/l, qe is amount of dye adsorbed at equilibrium in mg/g, KL (mg/l) and b (mg/g) are Langmuir constant, representing the maximum adsorption capacity for the solid phase and energy constant related to heat of adsorption. The constant b and KL can be evaluated from the intercept and slope of linear plot of the experimental data of 1/ qe vs 1/Ce respectively. The plot of of 1/ qe vs 1/Ce is shown in figure no.6.
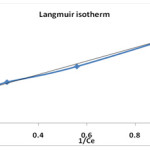 |
Figure 6: Langmuir plot for removal of sulphur on tendu leaves. |
Freundlich isotherm: Freundlich isotherm has been widely adopted to characterize the adsorption capacity of organic pollutants using different adsorbents by fitting the adsorption data. The Freundlich isotherm in its linearised form can be written as,
log qe = log Kf – 1/n × log Ce
Kf is a Freundlich constant related to the adsorption capacity (mg/g) and 1/n is the intensity of adsorption. The values of Kf and 1/n are determined from intercept and slope respectively from linear plot of ln qe v/s ln Ce. The 1/n values indicate the type of isotherm to be reversible (1/n=0), (0<1/n<1), unfavorable (1/n>). 26 The plot of ln qe v/s ln Ce is shown in figure no.7. The values of Kf and 1/n are calculated from graph shown in table no. 2. From experimental data the valueof 1/n is less than one indicating the given adsorption process is favorable.
Table No. 2: Adsorption isotherm parameters for adsorption of sulphur dye
| Langmuir Isotherm | Freundlich Isotherm | Tempkin Isotherm |
| KL(mg/l) b(mg/g) R2 | KF 1/ n R2 | A(l/mg) B(J/mol) R2 |
| 0.566 11.47 0.996 | 0.130 0.5 0.993 | 4.107 0.596 0.933 |
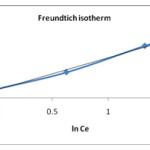 |
Figure 7: Freundlich plot for removal of sulphur on tendu leaves |
B is constant related to the heat of adsorption (J/mol) and A is Tempkin isotherm constant (L/mg). The constant A and B are calculated from slope and intercept of qe v/s ln Ce. The plot of of qe v/s ln Ce is shown in figure no.8. The parameters calculated from graph are shown in table no. 2. Based on correlation coefficient from all the three isotherm models, Langmuir model gave highest R2 value showing that the adsorption of sulphur dye on CATLR was best described by this model.
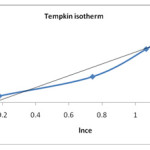 |
Figure 8: Tempkin plot for removal of sulphur on tendu leaves |
Conclusion
In the present study was carried out to examine reuse of tendu leaves waste from Bidi industry as a bio-sorbent material and results shown that it is used successfully for removal of sulphur dye from textile waste water. After adsorption pseudo second-order kinetics, Langmuir, Freundlich and Tempkin isotherm model is studied.
Activated carbon prepared from waste tendu leaves from local Bidi industries in Solapur city, India. Developing countries like India, industries cannot afford to use conventional waste water treatment like polyelectrolyte, hydrogen peroxide, activated carbon etc because these are economical. Therefore, preparation of biosorbent from zero cost tendu leaves waste is best alternative way of commercially available adsorbent for removal of dye from the textile waste water to prevent the environmental pollution.
Acknowledgments
I am grateful and thankful to Dr.S.D.Khambe, Department of Microbiology, Miraj Mahavidyalaya, Miraj. My sincere thanks to Nikhil Analytical and Research Laboratory, Sangli for analytical support for this research work.
References
- A. I. Ohioma, N.O. Luke and Odia Amraibure, “Studies on the pollution potential of waste water from textile processing factories in Kaduna, Nigeria”, J. of Toxicology and Environmental Health Sciences, Vol.1 (2), 34-37( 2009).
- S. R. Karmakar, “Chemical Technology in the Pre-Treatment Processes of Textiles”, ELSEVIER, 370-372 (1999).
- G. K. Nagda, V. S. Ghole, “Biosoption of Congo Red by Hydrogen Peroxide Treated Tendu Waste”, Iran. J. Envionmental Helth Sci. Engg, Vol.6 (3), 195-200 (2009).
- G. K. Nagda, V. S. Ghole, “Removal of Janus Green dye from aqueous solution by phosphoric acid carbonized agro-industrial waste”, Research Article, Science Asia,37, 38-42 (2011).
- S. m. Kanawade, R. W. Gaikwad and S. A. Misal “Low cost Sugarcane Bagasse Ash as an Adsorbent for Removal from Dye Effluent”, International J of Chemical Engg. And Applications, December,vol. 4, 309-318 (2010).
- V. K.Gupta, J. Ali and Saini V. K., “Adsorption studied on the removal of Vertiogo Blue 49 and orange DNAB from a waste material”, J. colloid Interface Sci, 315 87-93(2007).
- Kailash Daga, V. Pallavi and Santosh Chaudhary, “Removal of Nickel (II) by polyvinyl Alcohol Coated Carbon prepared from Datura Stramonium”, In. J. of Nature Environment and Pollution Technology, 2010, 9(1), 63-68.
- A. K. Jain, V.K. Gupta, A. Bhatnagar, J. Shubhi and Suhas, “A comparative Assessment of adsorbent preaped from industrial waste for the removal of cationic dye, J. Indian Soc., 80, 267-270 (2003).
- V. K. Verma, A.K. Mishra, “Kinetic and isotherm modeling of adsorption of Dyes onto Rice Husk Carbon” Global NEST Journal, 12(2), 190-196(2010).
- M. R. Gidde, Julie Dutta, Snehal Jadhav, “Comparative adsorption studies on Activated Rice Husk and Rice Husk Ash by using Methylene Blue as dye”, Int. congress on Environmental Reserch at Bits pilani Goa,1-11 (2008-09).
- F. A. Adekola and H. I. Adegoke, Adsorption of Blue dye on Activated carbons produced from Rice Husk, Coconut shell and cocout coirpith”, The J. of Science, 7(1), 151-157 (2005).
- Ola Abdel Wahab, Ahmed EL Nemr, Amany EL Sikily, Azza Khaled, “Use of Rice Husk for adsorption of Direct dyes from aqueous solution: A case study of Direct F. Scarlet,” EGYPTIAN J. OF AQUATIC RESEARCH, 31(1), 1-11(2005).
- Jyoti Sharma and Beena Janveja, “ A study on Removal of congored dyes from the effluents of textile industry using Rice Husk carbon activated by steam, “RJC-Rasayan J. Chem, , 1(4), 936-942(2008).
- P. Manikadan, K. Manjula Rani, S. V. Priya and V. N. Kowshalya, “ Adsorption kinetics for the removal of Hexavalent Chromium from Aqueous solution by Acid Activated Coal Fly ash (CFA)”, An Int. J. of Nature Environment and Pollution Technology, 2011, 10(1), 1-6.
- P. C. Mune, A. B. Bhosle, P. D. Deshmukh, C. M. Jangam, “Chromium Adsorption on activated carbon Derived from Tendu (Diospyros Melanoxylon) leaf Reuse: Influence of metal/carbon ratio, time, pH, Pelagia Research Library, Advances in Applied Sciences Research, 2010, 1(3), 212-221.
- G. K. Nagda, V. S. Ghole, “Utilization of lignocellulosic waste from Bidi Industry for removal of dye from Aqueous solution”, Int. J. Environmental Res., 2008, 2(4), 385-390.
- C. Prakash and S. Sivamani, “Removal of rhodamine-B,A basic dye by using tapioca peel activated carbon (TPAC)”, Pollution control, Colourage, , September Vol.LV No. 9, 61-64(2008).
- Poonam Motwani, Raj k. Vyas, Monika Maheshwari and Sangeeta Vyas, “Removal of Sulfamethoxazole from waste water by Adsorption and Photolysis, Int. J. of Nature Environment and Pollution Technology, 10(1), 51-58(2011).
- G. S. Gupta, “Treatment of waste water from Dye manufacturing using adsorption”, J. of Environment Science and Engg. , 8, 66-73(2010).
- H. Santapau, A. N. Henry, “A Dictionary of the flowering plants of India, CSIR, New Delhi.
- APHA, “Standard Methods for Examination Water and Waste Water. 21th edn, , Washington DC(2001).
- H. D. Chapmen, P. F. Pratt, “Methods of Analysis for Soil, Plant and waste water, Agricultural Sciences Publications, University of California, Berkcley.
- A.K.Krishnan, K. G. Sreejalekshmi and V. Sumol, “Adsorptive Retention of Citric Acid onto Activated Carbon Prepared from Havea baziliansis Sawdust: Kinetic and Isotherm Overview. Desalination, 257, 46-52.
- K. M. Joseph and B. T. Christelle, “Removal of Mercury(II) ions from Aqueous Solution using Granular Activated Carbon(GAC) and Kaolinite Clay from Mayouom in Cameroon: Kinetics and Equilibrium Studies”, Res. J. of Chemistry and Environment, 14(3), 60-65(2010).
- Poostchi Amir Ali, Mehrnia Mohammad Reza and Sarrafzadeh Mohammad Hossein, “Removal of dissolved organic carbon by multi-walled nanotubes, podered activated carbon and granular activated carbon”, Res. J. of Chemistry and Environment, 14(4), 59-66(2010).
- B. R. Puri, L. R. Sharma and M. S. Pathania, “Principles of Physical Chemistry”, 1152-1158(2002).
- A. S. Franca, S. O. Leandro and E.F. Mauro, “Kinetics and Equilibrium Studies of Methylene blue Adsorption by Spent Coffee Grounds, Desalination, 249, 267-272.

This work is licensed under a Creative Commons Attribution 4.0 International License.









