Volumetric Studies of Sodium Dodecyl Sulphate in Aqueous and Aqueous Amino Acid Solutions
Roksana Khatun1 and Md. Nazrul Islam2
1Department of Chemistry, Chittagong University of Engineering & Technology, Chittagong, Bangladesh.
2Department of Chemistry, University of Rajshahi, Rajshahi, Bangladesh.
Corresponding author: E-mail: ark19mrk@yahoo.com
The volumetric studies on the binary aqueous solutions of sodium dodecyl sulphate (SDS) and ternary SDS-amino acid-water systems have been carried out as a function of concentration and at 15, 20 , 25 , 30 , 35 , 40 , 45 and 50 °C. In this study the variation of apparent molar volume (fn) of SDS was observed in the low concentration region. The limiting apparent molar volume (f0n) values of SDS in aqueous amino acid solutions are greater than those in water and increase with the concentration at all the studied temperatures. The greater f0n value in amino acid solution has been demonstrated strong ion-ion interactions in the ternary system. Apparent molar expansions (FE) in aqueous solution and ternary SDS-amino acid-water systems are very sensitive to hydrophobic and hydrophilic solute-solute interactions. Expansibility, a and apparent molar expansion, FE reflect from the same structural alteration in the solutions. In our investigation, critical micelle concentration (c.m.c) of SDS at various SDS-amino acid-water systems also determined by apparent molar volume (fn). Among various physical parameters, apparent molar volume, limiting apparent molar volume, Apparent molar expansion has been recognized as a quantity that is sensitive to structural changes occurring in solutions.
KEYWORDS:Sodium dodecyl sulphate; glycine; alanine; apparent molar volume
Download this article as:| Copy the following to cite this article: Khatun R, Islam M. N. Volumetric Studies of Sodium Dodecyl Sulphate in Aqueous and Aqueous Amino Acid Solutions. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Khatun R, Islam M. N. Volumetric Studies of Sodium Dodecyl Sulphate in Aqueous and Aqueous Amino Acid Solutions. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23780 |
Introduction
Volumetric studies of SDS in aqueous and in aqueous amino acid provides great help to determine the nature of interactions and properties of this solution. And it is very important because of great applications of SDS in Pharmaceuticals and Biotechnological prosses. SDS represents a potentially effective topical microbicide, which can also inhibit and possibly prevent infection by various enveloped and non-enveloped viruses such as the Herpes simplex viruses, HIV, and the Semliki Forest virus.
In this paper, We reports the changes in the physical properties due to the mixing of water, sodium dodecyl sulphate & amino acid are thought of arising from hydrophobic interactions among solute and solvent molecules. Here has been used ternary process and the results of volumetric studies on the binary aqueous solutions of sodium dodecyl sulphate(SDS) and ternary SDS-amino acid-water systems gives vast information about volumetric properties. Here we used two amino acids (glycine & dl-alanine) and sodium dodecyl sulphate(SDS). Surfactant molecules (e.g. CTAB, SDS, TritonX-100, etc.) self-aggregate into super molecular structures when dissolved in water or oil. The simplest aggregate of these surfactant molecules is called a micelle; and the dispersion of the aggregates in water or oil is referred to as a micellar solution. A typical micelle has size of ~ 50 Å and is made of about 100 surfactant molecules. The surfactant molecule consists of two parts, namely, a polar hydrophilic head group and an apolar hydrophobic tail (hydrocarbon chain). The concentration above which micelle formation becomes appreciable is termed the critical micelle concentration (c.m.c).
Micelle formation is as typical hydrophobic process in water. In aqueous medium, surfactant molecules with their long hydrophobic tails undergo hydrophobic hydration. As the surfactant concentration increases, the association of surfactant molecules occurs by hydrophobic interaction and this result in the removal of the non- polar portion of the molecule from the external aqueous environment to form the interior of the micelle while the hydrophilic groups are exposed to the aqueous environment
Therefore, the present studies are to determined apparent molar volume, apparent molar expansion and critical micelle concentration (c.m.c) at low region concentration in binary and ternary system. A review of literature shows that only a few authors have attempted to determine of these properties in ternary system at low region concentration
Experimental
The Surfactant used in this study was sodium dodecyl sulphate(SDS). The c.m.c value of SDS in water reported in the literature value closely around 0.009m at 250C. The values of mean aggregation number of SDS vary from 58 to 64. Glycine (purity. Mass fraction ≥0.99), DL-alanine (purity. Mass fraction ≥0.99) procured from Fluka Chemical Company, Switzerland were used without further purification. Supplied distilled water was redistilled and deionized by passing through two ion exchange columns. The deionized water was distilled again in alkaline KMnO4 medium and used for preparation of solution. Conductivity of this deionized water was found to be 5×10-6 S.cm-1. An electric balance with an accuracy of ± 0.0001 g was used for weighting. The densitometer (DSA-5000, Anton Paar, Austria) was used for the measurements of density and ultrasonic sound velocity. The solutions were prepared by weight immediately before the measurement. The ternary solutions were prepared by mixing appropriate mass of the components. The amount of each component was later converted into their mole fraction. Precautions were taken to prevent the introduction of moisture into the experimental examples. Each time, the solution was prepared immediately before the density measurement.
Results and Discussion
Apparent molar volume
The apparent molar volume,øv , was calculated from the measured density of the solution using the relation
![]()
Where M2, m, ρ and ρ0 respectively the molar mass of solute, molality of solution, density of solution and density of solvent. Density data of solution and solvent are given in Table 1and the calculated values at different temperatures are given in Table 2 as a function of molality of the solutions. The plots of the against are presented in Figures 1-3.
For surfactant solutions physical properties such as, osmotic pressure, turbidity, electrical conductance and surface tension were used to determine the critical micelle concentration (c.m.c). In our investigation we tried to observe the variation of apparent molar volume of SDS as we focused our study in the low concentration region. The – plots show sudden change in value at a particular molality after which show linear change. The c.m.c of SDS in aqueous and aqueous amino acid solutions were determined by extrapolation method. The c.m.c values of SDS in aqueous and aqueous amino acid solutions are presented in Table 4
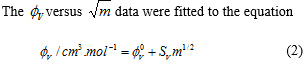
by least square method. The limiting apparent molar volumes, , of SDS are presented in Table 3.
Table 4 reveals that our c.m.c values of SDS in water are somewhat larger than the literature values (0.009). An increase in c.m.c values in glycine and alanine solution was observed which demonstrates that glycine and alanine behave as water structure breakers. It has been reported that addition of structure breakers causes an increase of cmc [1].
The viscometric study of Devine and Lowe [2] reports that glycine is water structure breaker whereas, alanine is water structure maker. The effect of concentration of glycine and alanine and that of temperature is not regular, the observed trend is that, c.m.c increases with the increase in amino acid concentration. c.m.c’s of SDS would be expected to increase with increasing temperature [1]. No such distinct behaviour is observed from the study. This may be due to the uncertainties associated with the measurement in very low concentration region. The uncertainty in measurement is greater than the expected variation of cmc values. Further attempts of cmc determination through measurement will establish this method.
It is evident from Figures 1-3 that values of SDS increase with the increase in solute concentration. The concentration dependence of in aqueous and mixed aqueous solutions has been explained in terms of the solute-solute interaction. The usual interpretation is that the solute species interact through the destructive overlap of their hydration spheres [3]. For apolar species, the positive volume contribution to , originated from the hydrophobic hydration starts decreasing as the solute concentration is increased. The overlap of two hydrophobic hydration cospheres relaxes some water molecules from the salvation sphere to the bulk giving rise to a negative change in volume. For hydrophobic ionic species the volume of water molecules is smaller in the salvation shell due to (a) the effect of electrostriction [4] and (b) a decrease in the hydrogen-bonded network of water molecules in the salvation sphere than in the bulk (the so called structure-breaking effect). Further, if the ions are oppositely charged, this type of interaction also causes an attraction since the orientation of water molecules by the cations leads to favorable anion-water dipole interactions (and vise versa). In this way even the electrostricted water molecules may be shared, resulting in a positive volume change. The structure-breaking influence of ionic species on the hydrophobic hydration sphere of apolar groups gives a negative volume effect
Anionic surfactant sodium dodecyl sulphate has a long hydrophobic chain and charge centre. The concentration dependence of of SDS can be interpreted in terms of the interactions involving the hydrophobic part as well as the charged centres. The values of SDS increase with the increase in molality i.e, Sv value positive. The positive sign of Sv indicates that interactions involving charged moieties dominate over the apolar group-apolar group and apolar group- charged centre interactions.
The values, obtained by fitting -data by least squares method are tabulated in Table 3. The values of SDS in aqueous amino acid solutions are greater than these in water and increase with the concentration at all the studied temperatures. The variation of with molality of amino acids can be rationalized in terms of the cosphere overlap model [5]. According to this model, the overlap of cosphere of ion (or a polar group ) with that of another ion (or a polar group) gives positive change in volume, while that of an ion (or a polar group) with that of a hydrophobic group and a hydrophilic group with another hydrophobic group results in a negative change in volume. As in the limit of infinite dilution the solute-solute interaction is minimal, the mutual overlap of the cospheres of zwitterionic amino acid with those of hydrophobic and hydrophilic group of SDS produces negative and positive changes in volume, respectively. The greater values in amino acid solution demonstrate strong ion-ion interactions in the ternary system. The relaxation of the electrostricted water molecules, due to strong localized ion-zwitterion interactions, from the cosphers of both the amino acid and SDS to the bulk causes an increase in volume , this increase in volume outweighs the volume decrease due to the overlap of cospheres of hydrophobic parts of SDS and amino acids. Overlap of cosphere of amino acids gives positive change in volume.
As the concentration of amino acid increases the zwitterions-SDS anion and also the amino acid interactions increase giving rise to an increased value. This in, and all probability account for the increase in value with the increase in amino acid concentration.
The of SDS in ternary solution can alternatively be thought of as arising from four constituents [6], as
=Vvw + Vf + Vh + Vs (3)
where, Vvw and Vf are van der Waalls volume [7] and volume of empty spaces present therein [8], respectively. The Vh and Vs represent the contributions due to the hydrophobic and hydrophilic hydration. The Vvw and Vf are assumed to be the same in aqueous amino acid as in water [9]. The variation of is, Therefore, due to the changes in (Vh + Vs) resulting from SDS-amino acid, amino acid-amino acids and amino acid water interactions; the contribution from water-water interactions is assumed to be negligible.
At the same amino acid concentration, the increases with the increase in temperature. This may be due to the resultant of the following effects;
Due to the increased thermal energy at higher temperature, the relaxation to the bulk of the electrostricted water molecules from the ionic interaction regions results in a positive volume change.
An increase in temperature renders the amino acid- amino acid interaction relatively weaker giving rise to a small negative volume changed.
A decrease in amino acid-water interactions causes an increased volume change..
 |
Table 1: Density (ρ) and ( ρ0) of SDS in water and aqueous amino acid solutions as a function of molality of SDS at different temperature.
|
 |
Table 2: Apparent molar volume (Фv/cm-3.mol-1) of SDS in aqueous and aqueous aminoacid solutions as a function of molality and different temperature. |
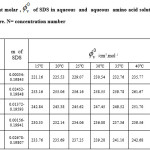 |
Table 3: Limiting apparent molar , of SDS in aqueous and aqueous amino acid solutions as a function of molality and temperature. N= concentration number |
 |
Table 4: Critical micelle concentration (c.m.c) of SDS in aqueous and aqueous amino acid solutions as a function of molality and different temperature. |
 |
Figure 1: Limiting apparent molar volume of SDS in water , water+glycine(1.3), dl-alanine (1.216) as a function of molality of SDS at different temperature; ♦-15оC, ■-20 оC, ▲-25 оC, ◊-30 оC, □-35 оC, ∆-40 оC, ●-45 оC, ○-50 оC. |
Apparent molar expansion and expansibility
Apparent molar expansion, ΦE was computed from measured density data using the relation
![]()
where, α0 is the expansibility of water taken from G. S. Kell [10], α is the expansibility of solution calculated using the equation
![]()
The parameters a1 and a2 were obtained by the regression of (ρ – ρ0) – T data according to equation
![]()
using the ai parameter the expansibility of solution was calculated from equation (5) and hence apparent molar expansion was calculated using equation (4). The calculated and ΦE as a function SDS molality and temperature are presented in Table 7. Graphical representation of and ΦE against molality is given in Figures 2-3. Apparent molar expansions in aqueous solution are very sensitive to hydrophobic and hydrophilic solute-solute interactions [11,12]. Expansibility, and apparent molar expansion, ΦE reflect from the same structural alteration in the solutions. One might, therefore, expect the same response due to change of other parameters like concentration, temperature, pressure, etc. Hence, we concern with apparent molar expansion only. It is observed from isotherms that is positive for all the studied aqueous SDS and increases with an increase in the size of hydrophobic side chain of SDS and temperature. Since the aggregates due to hydrophobic interactions in aqueous environment are more sensitive to temperature and more unstable than the aggregates of water-water around the hydrophobic moieties, in turns, than that of around hydrophilic centers and in normal water [13-15], the greater expansibility of the former would be expected.
The positive values of reveal that as an increase of molal concentration of SDS, the aggregation through hydrophobic moieties due to hydrophobic interactions are facile. The hydrophobic interactions are more dominant as the size of hydrophobic chain becomes larger [16-18]. The increase of values with the size of hydrophobic chain accounts the predominance of hydrophobic interactions in SDS with larger hydrocarbon backbon
![Table 5: α0 is the expansibility of water taken from G. S. Kell [10],](http://www.orientjchem.org/wp-content/uploads/2012/03/Vol28_Iss1_Rok_Vol_Tab5-150x115.jpg) |
Table 5: α0 is the expansibility of water taken from G. S. Kell [10], |
 |
Table 6: The parameters a1 and a2 |
 |
Table 7: Expansibility ( α ) and apparent molar expansion (ΦE) of SDS in water as a function of molality and different temperature. |
 |
Figure 2: Expansibility ( α ) of SDS in water, water+glycine(1.3), water+dl-alanine(1.216) as a function of molality of SDS at different temperature; ♦-15оC, ■-20 оC,▲-25 оC, ◊-30 оC, □-35 оC, ∆-40 оC, ●-45 оC, ○-50 оC. |
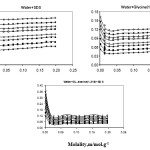 |
Figure 3: Apparent molar expansion (ΦE) of SDS in water, water+glycine(1.3), water+dl-alanine(1.216) as a function of molality of SDS at different temperature; ♦-15оC, ■-20 оC, ▲-25 оC, ◊-30 оC, □-3оC, ∆-40 оC, ●-45 оC, ○-50 оC. Click here to View figure |
References
- D.J. Shaw, “Introduction to Colloid and Surface Chemistry”, 4th Ed, Butterworth Heinemann 1991. 87
- W.Devine and B.M. Lowe, J. Chem. Soc (A). 1977, 2113.
- R.W. Gurney, “ Ionic process in solution”. Structure, thermodynamics and transpore processes”, R.A. Horne, Ed.Wiley-Interscience, New York(1972).
- F.J. Millero, “Water and Aqueous Solutions: structure, thermodynamics and transport processes “ R.A. Horne, ED. Wiley- Intersceience, New York, (1972).
- H.L. Friedmann and C.V. Krishnan, “Water: A comprehensive treatise”, Ed.F.Franks, Plenum Press, New York, 1973, vol. 3, ch. 1.
- A.K. Mishra and J.C. Ahluwalia, J. Phys. Chem. 1984, 88, 86.
- A. Bondi; J. Phys. Chem , 1959,58, 929.
- D.E. Goldsack and R. Franchetto, Can.J. Chem, 1978,56, 1442.
- R. Bhat and J. C. Ahluwalia, J. Phys. Chem, 1985, 89, 1099.
- G.S. Kell, J. Chem. Eng. Data 20(1) (1975), 97-105.
- S. Senkow, S.K. Mehta, G. Douheret, A.H. Roux and G. Roux-Desgranges, Phys. Chem. Chem. Phys. 4 (2002), 4472.
- J.E Desnoyers, G. Caron, R. de Lisi, D. Roberts, A. Roux and G. Parron, J. Phys. Chem. 87 (1983), 1397.
- R. L Mancera, M. Chalaris, K. Refson and J. Samios, Phys. Chem. Chem. Phys. 6 (2004), 94-102.
- A. K. Covington and P. Jones, “Hydrogen-bonded Solvent Systems” Taylor & Francis Ltd., London, (Ed.) (1968), 221.
- F. Franks,”Water” The Royal Society of Chemistry, London, (1983), 44.
- E. M. Huque, J. Chem. Ed. 66 (1989), 581.
- W. Kauzman, Adv. Protein Chem. 14 (1959), 1.
- F. Franks,”Water” The Royal Society of Chemistry, London, (1983), 41

This work is licensed under a Creative Commons Attribution 4.0 International License.









