Thermodynamic and Biological Studies of Some Bivalent Metal complexes with 2,4-Dihydroxy butyrophenone Oxime (DHBOX)
F. Rehman and Samya Mairaj
Department of Analytical Chemistry, Faiz-E-Am Degree College, Meerut.
The 2, 4 di-hydroxy butyrophenone Oxime (DHBOX) behaved as mono protic acid and proton was replaced by the metal ion during complex formation & two type of complexes viz 1:1 and 1:2 in the system. The stability constant of DHBOX with bivalent metal ion was calculated. The order of stability constant was in the order Cu(II)>Ni(II)>Mn(II) which is in the agreement with the Irving Williams order. The values of change in free energy (DG), enthalpy (DH) and entropy (DS) for ligand were calculated at different temperature. The effect of ionic strength and temperature on stability constant were also studied. The antifungal activity and antibacterial activity of different concentration of test compounds were measured by determining the growth of test fungi by dry weight increase method and by agar diffusion method against alternaria alternate & E. coli. The order of antimicrobial effect was observed the following order Cu(II)>Ni(II)>Mn(II).
KEYWORDS:Thermodynamic and Biological study; DHBOX
Download this article as:| Copy the following to cite this article: Rehman F, Mairaj S. Thermodynamic and Biological Studies of Some Bivalent Metal complexes with 2,4-Dihydroxy butyrophenone Oxime (DHBOX). Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Rehman F, Mairaj S. Thermodynamic and Biological Studies of Some Bivalent Metal complexes with 2,4-Dihydroxy butyrophenone Oxime (DHBOX). Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24066 |
Introduction
The nitrogen, sulpher donar compounds like oxime, semicarbazide, thiosemicarbazide have significance due to their wide application in industries, medicine, detection and determination of various metal ions1,2. The different type of phenones and their oximes have aroused considerable interest as regard to their chelating ability with transition metal ions3-8. Which are used as antiseptic9, germicide10, anthelmintics11, analgesic12, antituberculosis13 and shows antibacterial, antifungal14 and antiviral activity15.
It has been established that the above compound exhibits increased activity in the form of their metal chelates. The structure capable of providing metal chelates may, therefore be provided more potential. Besides their medicinal and biological activities, o- hydroxyl phenones, their derivatives and metal complexes have also been reported as excellent analytical reagent16.
The present communication deals with the thermodynamic studies of Mn(II),Ni(II),Cu(II) complexes with 2,4-Dihydroxy butyrophenone oxime at 27 ± 0.2º in 75 % (v/v) dioxane water medium at different ionic strength and at different temperature. The antifungal and antibacterial studies of ligand and their complex with Cu(II), Ni(II),Mn(II) is also calculated by using standard methods.
Experimental
The ligand (DHBOX) was prepared by standard method. The following sets of solution were titrated against standard carbonate free sodium hydroxide solution (0.05M) (I),0.05M HClO4 (II)(I) + 0.01 DHBOX and (III) (II) + 0.08 M Metal ion.
The apparent volume of 75% dioxane + 25 % water mixture (V/V) was multiplied by appropriate correction factor17 to obtain the real volume. Ionic strengths of 0.1, 0.05 and 0.01M were maintained by adding required amount of NaClO4. The pH measurements were made on a systronic 335 digital pH meter and corrected by using Van Uiteret and Hass equation18. The value of n, nH and pL were calculated by using standard equations19. The log KH values was obtained from the proton ligand formation
Curve (plot between nH and pH and the formation constant of metal complexes were obtained from formation curves (plot between n and pL). The thermodynamic formation constants (Table-1) were obtained by extrapolation of the observed formation constants to zero ionic strength on the graph between log of the stability constant and √ µ, where µ is ionic strength.
The valves of change in free energy (ΔG), enthalpy (ΔH) and entropy (ΔS) for ligand were calculated at three different temperature at µ=0.1 NaClO4 in 75% (V/V) water-dioxane medium (Table-2) using the following equations.
ΔG = -2.303 RT log Kµ =0
ΔH= 2.303 R× ( T2 × T1/T2 –T1) (log K´´- log K´)
ΔS= 2.303 log K + ΔH/T
The values of pKmH, θ and ΔH were obtained by the following equations
pKH-pKmH = C(t – θ)2 and ΔH= 2.303 × 10- 4 RT2 (t – θ)
Where, pKH= -logKH at t °c, pKmH= minimum pKH at 0 °c and C=constant (5× 10-1 deg-2).
The antifungal activity and antibacterial activity of different concentration of test compounds were measured by determining the growth of test fungi by dry weight increase method14 and by agar diffusion method. The test organism were alternaria alternate which was screened in vitro Richard’s liquid medium. The percentage of inhibition was also calculated (Table-3), while antibacterial activity was calculated against E.Coli (Table-4).
Result and Discussion
The ligand (DHBOX) behaved as a mono protic acid due to deprotonation of the phenolic OH group ortho to the keto group from which the proton was replaced by the metal ions during complex formation. This was evident from the fact that the metal titration curves were well separated from the ligand titration curves.
The values of n >2 were not obtained in any case showing thereby the formation of only type of complexes, viz 1:1 and 1:2 in the System. In the complexes of Mn(II), the value of n is always <1, which is due to hydrolysis, in such cases only log K1 values have been calculated. In the case of Ni(II) , the values of n- were < 1.5, hence the log K2 values were calculated using the equation
2logK = logK1 + logK2, where
logK= pL at n =1.0. It has been found that logK1 > logK2 in all the cases studied. The order of stability constants of bivalent metal complexes was found Cu(II)>Ni(II)>Mn(II) which is in the agreement with the Irving-Williams order20.
The more stability of Cu- complex in comparison to that of Ni complex may be attributed to the difference in their respective configuraton21. it is probable that Cu (II) forms planar chelates, while Ni (II) forms tetrahedral or preferable octahedral chelates.
The greater stability of Zn (II) chelates in comparison to those of Ni(II) may be attributed to the electron delocalization of 3d orbitals of Zn(II) through interaction with oxygen orbitals not taking part in π- bonding and the π- orbitals of benzene rings.
The value of log βn and log KH decreases with the increase of ionic strength.
According to Huckle, the activity of a metal ion for its interaction with other molecular species decreases with the increase in the ionic strength of the medium. Accordingly, the formation constant would decrease with increase in the ionic strength of the medium, which in agreement with the observation of Debye22. The Protonation constant of ligand and stability constants of metal complexes decrease with the increase in temperature.
The Screening results indicate that fungal and bacterial growth was inhibited on addition of chemicals at varying concentrations. However, the ligand as well as the metal complexes showed increased activity at higher concentration than at lower concentration
for a given fungus and bacteria.
The activity showed a gradual change with change of metal ion in the complexes and observed the following order.
Cu(II)>Ni(II)>Mn(II)
Thus,Mn(II)- complex is least toxic while Cu(II) complex has the maximum activity. This may attribute to the fact that copper itself is a toxic element and the increase in toxicity in the metal chelates is probably either due to the faster diffusion of the chelates as a whole through the cell membrane23 which may block the enzymatic activity of the cell or else it may catalyse toxic reactions among cellular constituents.24-26
Antibacterial agent exert their action on the pathogen in the following ways.
Inhibitors of cell wall synthesis.
Inhibitors of Bio-synthesis( i.e.inhibit production of purines,pyrimidine,AA, Vitamins,Protein, RNA,DNA).
Inhibitors of energy production (Inhibit the respiration or by uncoupling of oxidative phosphorylation (Disruption the metabolic activities).The experimental data demonstrated that chelation can increase antimicrobial activity than ligand. It has been suggested that metal chelation reduced polarity of metal ion mainly because of the partial sharing of its positive charge with the donar group & possibility of delocalization of d- election occuring with in the whole chelate ring system formed during coordination. This process of chelation thus increase the lipophilic nature of the central metal atom which in turn favours its permeation through the lipid layer of the membrane.
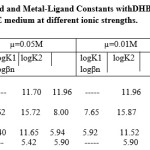 |
Table 1:Proton-Ligand and Metal-Ligand Constants withDHBOX at 27 ± 0.2° in 75% (V/V) DIOXANE medium at different ionic strengths. Click here to View table |
Table 2: Protonation Constants and the Thermodynamic Parameters at Different Temperature for DHBOX at µ = 0.05M.
| Temperature °K | PK1H | – ΔGK Cal/mole | – ΔHKcal/mole | ΔSCal/deg/mole | 0°C | PKmH |
| 293 | 12 | 16.11 | —– | 26.80 | —– | —– |
| 300 | 11.86 | 16.30 | 8.26 | 26.82 | 205 | 10.10 |
| 305 | 11.70 | 16.51 | —— | 26.81 | —– | —– |
Table- 3: Fungicidal screening data of DHBOX and their metal chelates against Alternaria Alternate at varying concentration.
| 0.10% cons. | 0.20% cons. | 0.30% cons. | 0.40% cons. | |||||
| Test.solution | weight | % inhibiton | Weight | %inhibiton | Weight | % inhibition | Weight | %inhibtion |
| Control | 1.20 | – | 1.20 | – | 1.20 | – | 1.20 | – |
| Fluconazole | 1.1664 | 2.8 | 1.143 | 4.78 | 1.12212 | 6.49 | 1.095 | 8.75 |
| HMBOX | 1.1604 | 3.3 | 1.134 | 5.52 | 1.098 | 8.49 | 1.071 | 10.75 |
| HMBOX-Mn(II) | 1.0992 | 8.4 | 1.1316 | 5.52 | 1.05504 | 12.08 | 1.0002 | 16.65 |
| HMBOX- Ni(II) | 1.0632 | 11.4 | 1.071 | 10.78 | 1.04232 | 13.14 | 0.9603 | 19.88 |
| HMBO- Cu(II) | 0.828 | 31.0 | 0.98 | 18.15 | 0.9273 | 22.72 | 0.8712 | 27.40 |
Table 4: Antibacterial Activity data of DHBOX and their metal chelates against E.Coli at varying concentrations.
| Test Solution | Inhibition Zone (mm) | |||
| 0.10% Conc. 0.20% Conc. | 0.30% Conc. | 0.40% Conc. | ||||
| Control | – | – | – | – |
| Ciproflaxacin | 5.64 | |||
| HMBOX | – | – | 4.24 | 6.82 |
| HMBOX-Mn(II) | – | 4.2 | 4.85 | 7.18 |
| HMBOX-Ni(II) | – | 6.6 | 7.16 | 10.40 |
| HMBOX-Cu(II) | – | 7.7 | 7.94 | 11.12 |
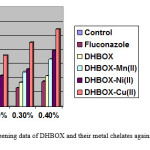 |
Figure 1: Fungicidal Screening data of DHBOX and their metal chelates against Alternaria Alternate at varying concentration. Click here to View figure |
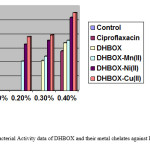 |
Figure 2: Antibacterial Activity data of DHBOX and their metal chelates against E.Coli at varying concentration. Click here to View figure |
References
- Winkelmana D.A., Brimke Y. and Petering D.H. : Bio-inorg. Chem. 1974,3,261.
- M. Akbar Ali, Livingstone S.E.: Coord. Chem. Rev 1974,13,101
- RehmanF., Rastogi S.N. and Jetley U.K.: J. Indian Chem. Soc. 1990,67,34
- Jetley U.K., Manu Shukla , Rehman F., Ja Singh, Sharma K. N. and Rastogi S.N. : J. nst. Chemists (India) 1987,59,91-94.
- Samya mairaj and Fazlur Rehman, Oriental j. of Chemistry 2011,27(1),221-225
- F.Rehman,Samya Mairaj and Manu Bhardwaj Oriental,j. of Chemistry 2011,27(3),1209-1214.
- Patel N.K.B., Desai K.K. : Asian J. of Chem. 2004, 16(2) 1076-80
- Sarita Sharma, Rameshwar and Mehta J.R. : Indian J. Chem. 1996,35,76-78
- Johnson T.P. and Fimslaine : J. Amer. Chem. Soc. 1921,43,348
- Fizikawa F., Sewaguish G. : J. Pharm. Soc. Japan 1952,72,1033
- Krotov A.I. and Bekhli : PharmakilI. Toksikol 1958, 21,49
- Entez Pubmed : Pubmed Indexed for medicine 2000,55,736-41
- Kunes J., Bazant J., Pour M., Waisser K., Slosarek M., Jaroter J. :
- Pubmed Indexed for medicine 2000, 55, 725-29
- Rehman F., Rastogi S.N., Jetely U.K., S. Asif Zaidly, Khan I. A.Oriental J. of Chem. 1988,4, 49-52
- Black Well Synergi : Applied Microbiol 2005, 40131-212
- Prakash D. C., Gupta A.K., Ramanandan Prasad, Yadav A.K..:Oriental J. of Chem. 2004, 20(1), 147-150
- Rao U. B. and Mathur H.B. : Indian J. Chem. 1969,7, 1234
- Van Uitert L.G. and Hass C.G.: J. Amer. Chem. Soc. 1953,3192
- Iriving H. and Rossotti H.S.: Chem. Soc. 1953,3397
- Irving H. and Williams R.J.P. : Chem. Soc. 1953,3192
- Jetley U.K., Manu Shukla, Rehman F., Jai Singh, Sharma K.N. and Rastogi S.N.: J. Inst. Chemists (India) 1987,59,91-94
- Sharma R.C., Tripathi B.P., Khanna S. and Sharma R.S. : Curr Sci.1981,50,784
- Debye P. Trans. Electrochim. Soc. 1942,7,82
- Horshall J.G. and Rich : Indo phytopathol 1953, 6, 1
- L.S.D. Yadav, S Singh., Indian J. Chem. 40B,40(2001)
- U.K.Jetley, Bibhesh k. Singh, Bhagwan S Gorg & Parashuram mishra: Journal of coordination Chemistry 2007, 60,2243-2245.

This work is licensed under a Creative Commons Attribution 4.0 International License.









