Theoretical Studies on Copper Extraction by means of Polymeric Membrane Contactors
Azam Marjani* and Saeed Shirazian
Islamic Azad University, Arak Branch, Department of Chemistry, Arak (Iran).
Solvent extraction of copper in hollow-fiber membrane contactors was investigated in this study. Extraction of copper is carried out conventionally using mixer-settlers. These contactors have some disadvantages such as consumption of high energy, dispersion of two phases and high operating costs. Membrane contactors can obviate the need for conventional contactors. The most favorable module used as membrane contactor is hollow-fiber module. These modules provide high surface area per contact volume. A known interface can be established between aqueous and organic phases for the purpose of mass transfer and extraction. Equilibrium is established at the aqueous-organic interface and depends on the hydrophobicity or hydrophilicity of the membrane. Investigations on copper extraction revealed that these contactors can be used as a new device for extraction of copper. Some authors have studied extraction of Cu2+ with a kerosene solution of di(2-ethylhexyl) phosphoric acid (D2EHPA) using hollow-fiber membrane contactors.
KEYWORDS:Mass transfer; Copper extraction; Membrane contactor; Solvent extraction
Download this article as:| Copy the following to cite this article: Marjani A, Shirazian S. Theoretical Studies on Copper Extraction by means of Polymeric Membrane Contactors. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Marjani A, Shirazian S. Theoretical Studies on Copper Extraction by means of Polymeric Membrane Contactors. Available from: http://www.orientjchem.org/?p=11789 |
Introduction
Due to increasing copper world demand, there is a strong need for copper extraction from low grade ores. Extraction of copper from acidic solutions is an important process in hydrometallurgy and chemical industries. The most common process for extraction of copper is copper Leaching, Solvent Extraction and Electrowinning circuit (LX–SX–EW) (see Fig. 1) [1]. This process is currently used in extraction units. Studies on the LX-SX-EW process have mainly focused on the process equipment and control of the plants. Appropriate design and control of the plant would keep the process close to the optimum, thus increasing the amount of copper produced and reducing the amount of chemicals and energy costs [2]. The heart of plant is extraction of copper from acidic solution. To do this, aqueous phases containing copper ions come into contact with an organic phase containing extractant. By contacting the aqueous and organic phases, a reaction occurs that causes formation of extractant-copper complex [3-8].
![Figure 1: Typical LX–SX–EW copper plant [1].](http://www.orientjchem.org/wp-content/uploads/2012/03/Vol28_Iss1_Aza_The_Fig-1-150x150.jpg) |
Figure 1: Typical LX–SX–EW copper plant [1]. Click here to View figure |
Mixer-settlers types used in commercial copper extraction
Mixer-settlers are common devices which are used as extraction medium for solvent extraction. These devices are used as contactor between feed and extractant phases. Mixer-settlers first were utilized in the nuclear industry because of their low headroom. This advantage minimizes the amount of shielding required. Other advantages of mixer-settlers include simplicity and low maintenance costs. Typically, the nuclear mixer-settlers consisted of partitioned “boxes” to avoid interstage piping, and relied on density differences to provide driving force for liquid flows. These advantages made the mixer-settlers a good choice for SX circuits in uranium ore processing plants in the nineteen fifties and sixties followed by the initial copper SX plants in the late nineteen sixties and early seventies. Since then the use of mixer-settlers has been standard practice for copper extraction due to their simplicity, ease of operation, stability under a wide range of flows, accessibility for clean-out, reliable scale-up and suitability for very large scale plants. Much has been done to improve efficiency and reduce capital and operating costs for the basic design, which has led to the development of new devices [2, 9-10].
Hollow-fiber membrane contactors
Generalities on membrane contactors
Membrane contactors (MCs) are devices that are employed to keep in contact two specific phases. Membrane contactors do not offer any selectivity for a particular species with respect to another, but simply act as a barrier between the phases involved, by allowing their contact in correspondence of a well defined interfacial area [11]. In membrane contactors there is no mix of phases and thus dispersion phenomena do not occur. The species are transferred from one phase to the other by only diffusion. The membranes are usually microporous and symmetric and can be both hydrophobic and hydrophilic [11].
In hydrophobic membranes, the membrane can be wetted by non polar phases (e.g., non polar organics) or filled by gas, while the aqueous/polar phase cannot penetrate into the membrane pores (see Fig. 2).
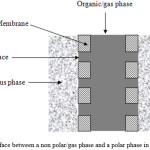 |
Figure 2: Interface between a non polar/gas phase and a polar phase in a hydrophobic membrane. |
In this way, it is possible to define the area of contact in correspondence of the pores mouths. In order to avoid the mixing of the two phases, it is important to carefully control the operating pressures. First of all, the pressure of the aqueous/polar phase has to be equal to or higher than the pressure of the wetting/filling phase. This permits to eliminate any possibility of dispersion as drops of one phase into the other phase. Moreover, the interfacial area can be established at the pore mouth only if the penetration of the aqueous/polar phase into the membrane pores is prevented. The hydrophobicity of the material is not, in fact, a warranty for keeping the pores aqueous/polar phase-free. If a critical value of pressure, called generally breakthrough pressure, is exceed, the membrane loses its hydrophobic character and the aqueous/polar phase starts to wet it [11].
In hydrophilic membranes, the aqueous/polar phase wets the membrane pores while the non polar/gas phase is blocked at the pore mouth. In this configuration the interface is established at the pore mouth at the non polar/gas phase side and the dispersion as drops between the phases is avoided by working with pressures of the non polar/gas phase equal to or higher than the wetting phase pressure (Fig. 3).
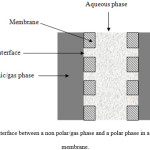 |
Figure 3: Interface between a non polar/gas phase and a polar phase in a hydrophilic membrane. Click here to View figure |
Hollow-fiber membrane contactors (HFMCs)
Hollow-fiber contactors have received the most attention. Hollow-fiber modules targeted for industrial applications (as opposed to medical ones, e.g., blood oxygenation) are available from a variety of sources.
The most well-known module designed for concentration-driven mass transfer is the Liqui-Cel Extra-Flow module offered by CELGARD LLC (Charlotte, NC; formerly Hoechst Celanese), shown in Fig. 4. This module uses Celgard microporous polypropylene fibers that are woven into a fabric and wrapped around a central tube feeder that supplies the shell side fluid. Woven fabric allows more uniform fiber spacing, which in turn leads to higher mass transfer coefficients than those obtained with individual fibers. Typically, the fibers inside diameter and wall thickness are 240 and 30 mm, respectively. The fibers are potted into a solvent-resistant epoxy or polyethylene tube sheet, and the shell casing is polypropylene, PVDF or 316L stainless steel [12].
![Figure 4: Hollow-fiber membrane contactor of Celgard LLC [13].](http://www.orientjchem.org/wp-content/uploads/2012/03/Vol28_Iss1_Aza_The_Fig4-150x150.jpg) |
Figure 4: Hollow-fiber membrane contactor of Celgard LLC [13]. Click here to View figure |
As shown in Fig. 4, the Extra-Flow module contains a central shell side baffle, a feature which offers two advantages. First, the baffle improves efficiency by minimizing shell side bypassing; second, it provides a component of velocity normal to the membrane surface, which results in a higher mass transfer coefficient than that achieved with strictly parallel flow. The smallest Liqui-Cel modules are 21/2 inches in diameter and contain 1.4 m2 of contact area, while the largest are 10 in. in diameter and offer 130 m2 of contact area by virtue of 225000 fibers [11, 12].
Advantages of membrane contactors include the following:
The available surface area remains undisturbed at high and low flow rates because the two fluid flows are independent.
Emulsion formation does not occur, again because there is no fluid/fluid dispersion.
Unlike traditional contactors, no density difference is required between fluids.
Scale-up is more straightforward with membrane contactors.
Modular design also allows a membrane plant to operate over a wide range of capacities.
Interfacial area is known and is constant, which allows performance to be predicted more easily than with conventional dispersed phase contactors.
Solvent holdup is low, an attractive feature when using expensive solvents [11].
Solvent extraction of copper using hollow-fiber membrane contactors (HFMCs)
Extraction of Cu2+ is carried out mostly with organophosphorus extractants such as di(2-ethylhexyl)phosphoric acid (D2EHPA) in hydrometallurgical and waste water treatment processes [14, 15]. Conventional copper extraction processes are carried out using equipments such as packed towers, mixer-settlers, etc. which try to maximize the contact area of two phases for mass transfer operation. Hollow-fiber membrane contactors can overcome the drawbacks of conventional Cu2+ extraction processes. This process is so called membrane based solvent extraction (MBSE). Two membrane modules are needed. The first one is for extraction and the second one is for stripping and recovery of extractant. At the first module, aqueous feed containing copper ions is contacted with the extractant phases. At the second modules so called membrane bases solvent stripping (MBSS), the extractant is recovered and recycled to the extraction module [14].
Chemistry of solvent extraction
The extraction of divalent heavy metals such as Cu2+ from sulfate solutions with D2EHPA dissolved in kerosene can be expressed as follows [16]:
![]()
where the overbar refers to the organic phase and (HR)2 represents the dimeric form of D2EHPA. The extraction distribution coefficient and equilibrium constant Kex are given by:
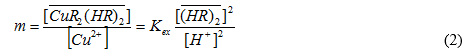
where is the distribution coefficient.
Fig. 5 shows concentration distribution of solute in the membrane contactor. Three mass transfer resistances are clearly shown.
![Figure 5: Concentration distribution in the membrane-based solvent extraction [17-19].](http://www.orientjchem.org/wp-content/uploads/2012/03/Vol28_Iss1_Aza_The_Fig5-150x150.jpg) |
Figure 5: Concentration distribution in the membrane-based solvent extraction [17-19]. Click here to View figure |
Two operational modes are applied for solvent extraction using hollow-fiber membrane contactors including one through and recirculation modes. Extraction efficiency is higher in recirculation mode. Juang et al. [17] studied the extraction of copper from acid solution using a HFMC. Their results revealed that by choosing proper operational conditions, complete recovery of copper from aqueous solution is possible. The results of their work indicated that flow rates of feed and solvent phase are two important parameters which affect the removal efficiency of the process. They used a polypropylene hollow fiber contactor. Polypropylene is hydrophobic, therefore organic phase can wets the membrane pores and interface is formed in the aqueous side.
Conclusions
Extraction of copper can be carried out using either conventional contactors or hollow-fiber membrane contactors (HFMCs). The most favorable conventional contactor which is currently used for extraction of copper from aqueous solutions is mixer settler. The main advantages of mixer settlers include: strong operational loads, easy operation and maintenance, simple start-up. However, the main disadvantage of theses contactors is high operating cost and energy intensive. Recently hollow-fiber membrane contactors have emerged as powerful alternative for extraction of copper. These modules provide high surface area per contact volume. Interface between two phases can be determined easily. Equilibrium is established at the aqueous-organic interface and depends on the hydrophobicity or hydrophilicity of the membrane. Studies on membrane-based solvent extraction show that these contactors are effective in the removal of copper. Some authors have studied extraction of Cu2+ with a kerosene solution of di(2-ethylhexyl) phosphoric acid (D2EHPA) using hollow-fiber membrane contactors. The results show that membrane contactors are efficient in the extraction of copper and can provide complete copper recovery.
References
- C.M. Moreno, J.R. Pérez-Correa, A. Otero, Dynamic modelling of copper solvent extraction mixer–settler units, Minerals Engineering 22 (2009) 1350–1358.
- Tiina Komulainen, Pertti Pekkala, Ari Rantala, Sirkka-Liisa Jämsä-Jounela, Dynamic modelling of an industrial copper solvent extraction process, Hydrometallurgy 81 (2006) 52–61.
- Archana Agrawal, M.K. Manoja, S. Kumari, D. Bagchia, V. Kumar and B.D. Pandey, Extractive separation of copper and nickel from copper bleed stream by solvent extraction route, Mineral Engineering 21 (15) 2008, 1126-1130.
- Feng Xie, David Dreisinger, Copper Solvent Extraction from Waste Cyanide Solution with LIX 7820, Solvent Extraction and Ion Exchange 27 (4) 2009, 459-473.
- Zdravka Lazarova, Madlena Lazarova, Solvent Extraction of Copper from Nitrate Media with Chelating LIX-Reagents: Comparative Equilibrium Study, Solvent Extraction and Ion Exchange 23 (5) 2005, 695-711.
- N. E. El-Hefny, J. A. Daoud, Extraction of Copper(II) by CYANEX 302 in Kerosene from Different Aqueous Media, Solvent Extraction and Ion Exchange 25 (6) 2007, 831-843.
- “Krebs and Solvent Extraction” by R. Williams and A. Sonntag, presented at ALTA Copper Hydrometallurgy Forum 1995.
- “Design of Mixer-Settlers to Maximise Performance” by G. Miller, presented at ALTA Copper 2006.
- Dimiter Hadjiev, J.B.A. Paulo, Extraction separation in mixer–settlers based on phase inversion, Separation and Purification Technology 43 (2005) 257–262.
- P. Navarro and F.J. Alguacil, Extraction of copper from sulphate solutions by lix 864 in escaid 100, minerals engineering, vol. 12, no. 3, pp. 323-327, 1999.
- E. Drioli, A. Criscuoli & E. Curcio, Membrane Contactors: Fundamentals, Applications and Potentialities, Membrane Science and Technology Series 11, Elsevier, 2006.
- A. Gabelman, S. T. Hwang, Hollow fiber membrane contactors, J. Membrane Sci. 159 (1999) 61-106.
- A. Sengupta, CELGARD LLC Corporation, personal communication, 5 August 1997.
- S. Schlosser, R. Kertesz, J. Martak, Recovery and separation of organic acids by membrane-based solvent extraction and pertraction: An overview with a case study on recovery of MPCA, Sep. Purif. Technol. 41 (2005) 237–266.
- Johnston, B.E., Commercial applications of phosphorus-based solvent extractions. Chemistry and Industry (London), 1988(20): p. 656-660.
- Juang, R.-S. and H.-L. Huang, Mechanistic analysis of solvent extraction of heavy metals in membrane contactors. Journal of Membrane Science, 2003. 213(1-2): p. 125-135.
- Juang, R.-S., J.-D. Chen, and H.-C. Huan, Dispersion-free membrane extraction: case studies of metal ion and organic acid extraction. Journal of Membrane Science, 2000. 165(1): p. 59-73.
- Guo, J.-J. and C.-D. Ho, Theoretical study on membrane extraction of Cu2+ with D2EHPA in laminar flow circular tube modules. Desalination, 2008. 233(1-3): p. 247-257.
- Shirazian, S., A. Moghadassi, and S. Moradi, Numerical simulation of mass transfer in gas-liquid hollow fiber membrane contactors for laminar flow conditions. Simulation Modelling Practice and Theory, 2009. 17 (4): p. 708-718.

This work is licensed under a Creative Commons Attribution 4.0 International License.









