The Synthesis of a New Reagent for Detecting Cyanide
Erdong Liu
College of Chemistry and Chemical Engineering, Qufu Normal University, Shandong Province, China.
This chemical sensor method was selected to detect cyanide in this paper. We synthesized a new reagent which can produce color change from colorless to yellow when encountering cyanide. According to this feature, this reagent can be used to identify cyanide. This reagent had also a good ability of anti-interference, the color change is not obvious when this reagent Encounter the interference ions of F- , Cl- , Br- , I- , No3 - . So even though these ions are present with Cn- at the same time, this reagent can also identify cyanide accurately.
KEYWORDS:Cyanide; Synthetize; color change; chemical sensor
Download this article as:| Copy the following to cite this article: Liu E. The Synthesis of a New Reagent for Detecting Cyanide. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Liu E. The Synthesis of a New Reagent for Detecting Cyanide. Available from: http://www.orientjchem.org/?p=11786 |
Introduction
Cyanide is a molecule containing cyano group (-C≡N) which is unstable and can easily be broken down or transformed even in normal light and temperature 1-3. Cyanide is a class of highly toxic materials, commonly hydrogen cyanide, sodium cyanide, potassium cyanide, calcium cyanide, acetonitrile, acrylonitrile and other inorganic or organic substance. They are not the substance that chemists created; on the contrary, they are widely found in nature, especially in the biosphere. Cyanide exists in a wide variety of organisms, including photosynthetic bacteria, algae, plants and animals. Cyanide ion was first isolated by the Swedish chemist Cheel in 1872 and widely used in metallurgy, electroplating, printing, dyeing and agricultural fields. Cyanide can pass into the body by the digestive tract, respiratory tract, skin and other ways, causing vomiting, cramps, loss of consciousness and even death. After the decomposition of cyanide in the human body Cn– Emerge which can affect the body’s multiple organs and tissues, including blood circulation, vision, central nervous system, endocrine system and digestive system4-5. Based on the special nature of cyanide, environmental management in many countries has developed a rigorous cyanide management standard. U.S. Environmental Protection Agency (USEPA) proposed that the highest cyanide concentrations in drinking and ecological water are 0.05mg/L and 0.20mg/L. World Health Organization provided the highest level of Cn– in drinking water which is 0.07mg/L6-7. In order to protect the environment and the personal safety, the detection of cyanide ion is very important.
Currently tests for cyanide mostly are spectrophotometry, gas chromatography, ion chromatography, titration analysis and electrochemical analysis method. Most of these methods require sophisticated instruments, complex programs and a longer time. Optical sensor hasn’t these above problems. As the method is simple with convenient equipment, the determination results can be obtained quickly. After reacting with Cn–, the optical sensor can produce significant color change or fluorescence change, and sometimes the naked eye can determine the measurement results. Since chemical sensor has many advantages, this method has been applied to detect the Cn– in thispaper. We synthesize a new reagent, which can produce the color change from colorless to yellow when it encounters Cn–. And by testing for many interference ions, we find these ions do not produce significant color change. Therefore, the synthesized reagent can be used to determine Cn– by the color change.
In this paper, 2-amino-10-ethyl-acridone was selected to synthetize a new reagent which was used to identify Cn–. 2-amino-10-ethyl-acridone is a fluorophore of very high quantum yield. When a variety of recognition sites is introduced to the amino, the optical properties of subject molecules change easily through the reaction with object ions. Trifluoroacetic acid groups can react with the Cn–, so it was introduced to 2-amino-10-ethyl-acridone. 2-trifluoroaceticamino-2-amino-10-ethyl-acridone and the reaction process is as follows:
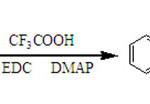 |
Scheme 1 Click here to View scheme |
Theoretically, once the trifluoroacetic acid groups of 2-trifluoroaceticamino-2-amino-10-ethyl- acridone react with Cn–, the optical properties of 2-trifluoroaceticamino-2-amino-10-ethyl-acridone will change. The study showed that the solution color changed from nearly colorless to yellow when the Cn– of 1×10-3mol/L was added to 2-trifluoroaceticamino-2-amino-10-ethyl-acridone of 5×10-5mol/L. This phenomenon verified the theory and showed that when there is only Cn– in solution, the synthetic reagent can identify Cn–. After testing the possible interference ions of F–, Cl–, Br–, I–, No3–, we found that the color change inconspicuous when adding these ions into the synthetic reagent. This phenomenon indicates that even if the solution contains these interfering ions, the synthetic reagent can also identify the Cn– selectively.
Experimental
Reagents and apparatuses
The reagents are 2-amino-10-ethyl-acridone (prepared in the laboratory), trifluoroacetic acid, edc, dmap, acetonitrile, distilled water, Nacl, and Dichloromethane.
The apparatuses are 50ml round bottom flask, 10ml volumetric flask, pipette, magnetic stirrer, and ultrasonic cleaner.
Test methods
Accurately weigh 2-amino-10-ethyl-acridone, edc, dmap, place them in a 50ml round bottom flask, and then add 10ml dichloromethane into it. Take the flask into the ultrasonic cleaner and turn on the power button. Add trifluoroacetic acid into the flask after the solution completely dissolves. Filter the reaction solution and wash precipitation with two distilled water and let it air-dry. Accurately weigh an amount of the product, make up a solution of 5×10-4mol/L. Separately pipette 1ml from this solution and place it in several test tubes. One tube is used as blank control, the rest are respectively added the solutions of Cn–, F–, Cl–, Br–, I–, and No3–. Then add 95% acetonitrile until the solution’s total volume reaches 4ml. Shake the test tube slightly to mix the solution well and then observe the color change. Also take a test tube, add 95% acetonitrile solvent to 4ml and use it as another reference solution.
Results and Discussion
Chromatography in the synthesis process
The sample is determined by HPLC with a mobile phase of acetonitrile and wate in the initial and latter stage of the reaction. Figure 1 shows the chromatogram of the initial reaction, and Figure 2 shows the chromatogram of the latter reaction. It can be seen from the two Figures that the peak at about 7 minutes appears significant change. From this phenomenon we can speculate that it is the product peak. Since the product is rare early in the reaction, this peak is small in Figure 1. After the reaction, the product is much more and the reactants are few, so we can see from the Figure 2 that the product peak is great and the peaks in front of it are small. The result of chromatographic identification is consistent with theoretical speculation.
 |
Figure 1: the chromatogram in the initial reaction |
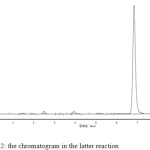 |
Figure 2: the chromatogram in the latter reaction |
The detection of cyanide ion
The test which contained the prepared solution was lined up to observe color. The left-most two tubes contained solvent and product solutions. The other tubes contained the product and testing ions, from left to right the added ions being Cn–, F–, Cl–, Br–, I–, and No3–. Figure 3 is the photo took in the experiment. As can be seen from the figure, the solvent is nearly colourless, the solutions of product and interfering ions are slight yellow, and only the solution of the product and Cn– is obviously yellow. This phenomenon is consistent with theoretical results and Cn– can be identified from this color change. Since this color change is not obvious, in order to get better results the experiment was repeated with changed conditions. Reduce the concentration of the product 10 times and increase the concentration of testing ions twice. The result shows that the solution of the product and Cn– is significantly yellow, while the other solutions are nearly colorless. The color change from colorless to yellow is very obvious. According to this phenomenon, Cn– can be easily identified and the ions of F–, Cl–, Br–, I–, No3– don’t interfere with the detection.
 |
Figure 3: the picture of the prepared solutions Click here to View figure |
Table 1: the color of each solution
|
composition |
solvent |
reagent |
reagent +Cn– |
reagent + F– |
reagent + Cl– |
reagent + Br– |
reagent + I– |
reagent + No3– |
|
color |
colourless |
pale yellow |
yellow |
pale yellow |
pale yellow |
pale yellow |
pale yellow |
pale yellow |
Conclusion
A new reagent was synthesized in this research, which can produce a significant color change when encountering Cn–. According to this feature, this reagent can be used to identify Cn–. Moreover, when the reagent encounters the ions of F–, Cl–, Br–, I–, and No3–, the color doesn’t change obviously, so it has certain ability of anti-interference. Cyanide is highly toxic substances, and the identification of Cyanide is important in many areas. The synthesis of the reagent provides a new method for the identification of Cyanide.
References
- Ding Zengzhi, Improvement of the method for determination of cyanide in drinking water, Preventive Medicine Tribune,17(6)527-528 (2011).
- Zhou Hong, Determination of cyanide in drinking water by flow injection method, Occup and Health, 27(16):1843-1845 (2011).
- Yu Haibo, A ratiometric fluorescent probe for cyanide: Convenient synthesis and theproposed mechanism, Sensors and Actuators B, 148 (5):110-116 (2010).
- Fu Mei, The synthesis and application of a new thiocyanate sensor based on naphthalimide, Chinese Journal of Luminescence, 30(4): 482-486(2011).
- Zheng Wei, Research progresses in bio-treatment of wastewater containing cyanide,Environmental Protection of Chemical Industry, 31(2):123-128 (2011)
- Wang Yong, Determination of cyanide in blood by ion chromatography, Modern Scientific Instruments, 4(4):69-71(2011).
- Zeynep Ekmekci, A monostyryl-boradiazaindacene (BODIPY) derivative as colorimetric and fluorescent probe for cyanide ions, Organic Letters, 10(3):461-464(2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









