Synthesis New and Novel Aryl Thiazole Derivatives Compounds
Abolfath Akbarzadeh1, Reza Soleymani 2*, Milad Taheri1 and Maryam Karimi-Cheshmeh ali3
1Department of Chemistry, Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
2Young researchers club, Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
3Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
Corresponding author: E-mail address: reza.soleymani@hotmail.com
Six new compounds of Thiazoles family was synthesis from condensation reaction of some compounds from pyrazolines. NMR, IR and elemental analysis was used for identification of these compounds. Position of aliphatic and aromatic hydrogens in pyrazolone and thiazole cycles had established proceeding of identification. Most of obtained compounds like aciform crystals, their color is white and yellow. Reaction time and yield of these compounds had shown better results as compared with other thiazole derivation
KEYWORDS:Thiazoles; Synthesis; NMR; Elemental analysis
Download this article as:| Copy the following to cite this article: Akbarzadeh A, Soleymani R, Taheri M, ali M. K. C. Synthesis New and Novel Aryl Thiazole Derivatives Compounds. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Akbarzadeh A, Soleymani R, Taheri M, ali M. K. C. Synthesis New and Novel Aryl Thiazole Derivatives Compounds. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23776 |
Introduction
Thiazoles are 5-membered cyclic compounds that nitrogen and sulfur group at different situation1. This compound have a lot of derivations and because of its derivations type have a lot of application in various industries, medical science and pharmacy. In 1887, Hantzsch et al identified and established molecular structure of thiazoles2. In 1889, the first derivation of thiazoles identified by Prop et al and was named 2-amino thiazole 3. After that until now, many derivations identified and reported4-9. However, identification of vitamin B1 and penicillin former components. It was found that thiazoles system including of S-C-N bound and have biological specifications. So, synthesis of thiazole derivations was become more important. Thiazoles are applicable in anti-fire and hard PVC stabilizer compounds. However, their derivations are applicable in anti-wart10, anti-allergenic11, anti-bacterial12, anti-spasm13, anti-paroxysm14 and anti-fungal15-17. Derivations of this compounds like 2-mercapto-5-hydroxy ethyl-4-methyl thiazole was used for destroying of helminthes and parasites and 2-methyl mercapto-4,5-di-4-methoxy as a drug for treatment of rheumatic. 2-aryl mercaptos are applicable for treatment of phlegmasia, too. 5-nitro-2,2-thio bis thiazole was used as an anti bacterial compound. But quality of substitution mechanisms is different in thiazoles group18. For example, substitution of methyl group in thiazoles structures is done by heteroatomic and hyper conjugation models. It is done by changing of pay electron. In this research, some new family of these compounds was produced that have many applications in medical science, pharmacy and industries.
Experimental Details
General method
All of chemical materials were purchased from Germany firm Merk. For this study, all of instruments that were used, are as following: microwave oven model LG-SOLARDOM LF-5901WCR, Japanese weighing with 0.100 g accuracy, model ANDGF-300, hitter and stirrer model Heidolphm Rn3004 Safty, Merk thin layer chromatography sheets Art no: 1:0554, Electro thermal melting point measurement apparatus, Memmert (oven) materials and glossy instruments desiccators (oven), vacuum pump Emerson model: C55-JJXH4205, Rmp: 1425/1725, Heidolph model: Labrota rotary solution. 1H-NMR spectra studying done by Bruker Avance 300 spectrometer with processing software XWINNMR version 3.1. FT-IR by a Perkin Elmer spectrum 1420 spectrometer in the frequency range of 4000–400cm−1 using KBr discs are reported on 1 scale. The IR spectra were recorded at room temperature at the spectral resolution of 1 cm−1. Used Glossy instruments are as follows: one and two ports round- bottom emery-top flasks species, simple, bubble and spiral radiator, Erlenmeyer flask, beakers, three and two ports, links, addition funnel, Buchner funnel, capillary tube and etc.
Synthesis
Synthesis methods and proceeding of this family from thiazoles was described at following. NMR, IR and elemental analysis was used for identification of these compounds.
Preparation of Ammonia gas
In the libratory, ammonium gas was prepared from reaction of ammonium chloride by sodium hydroxide. So, ammonium chloride and sodium hydroxide was heated. Sodium chloride salt, H2O and ammonium gas was prepared. For elimination of moisture, Calcium oxide was put in the path of ammonia gas exodus.
Preparation of ammonium di-thio carbamate
100.0 ml 95% ethanol was poured in 250.0 ml Erlenmeyer. It was kept in the ice bath and then was transited ammonia gas until weight increasing approximately 39.0 g was obtained. 60.0 ml (1.0 mol) carbon disulfide and 200.0 ml diethyl ether was added to the solution and it was kept for 2-3 hours in the ice container and then for 1 day in the refrigerator. After passing this time, yellow crystals of ammonium di-thio carbamate was formed. Then content of container was strained and washed by ether (50.0 ml). 80.0 g ammonium di-thio carbamate was produced.
Synthesis of 4-Bromophenacylbromid structure (1a)
50.0 g (0.2 mol) 4-bromo acetophenone was solved in 100.0 ml pure acetic acid in the 250.0 ml Erlenmeyer and then 12.5 ml (0.2 mol) bromine was added slowly to obtained solution and during the mixing, temperature of reaction container was kept to less than 20°C. Aciform crystals were produced approximately 30 minutes after adding of bromine. Mixing was continued for 30 minutes later. Then content of Erlenmeyer was strained and dried. 50.0 g production by 80% yield was produced. Obtained precipitation was recrystallized by 95% ethanol for purification. 4-bromophenacylbromide was produced by 108-109 °C melting point. TLC chromatogram and comparison of its Rf by bromo acetophenone Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (8:2 cyclo hexane–acetone) to give (56.0 g , 80%); A white solid; Mp: 108-109; IR (KBr): 3065-2950-2064-1693-1180-644; 1H-NMR (400 MHz, CDCl3) δ 4.44(s, 2H, CH2Br), 7.66-7.68 (d, 2H, 2-H, 6-H), 7.87-7.90 (J=9 Hz, d, 2H, 3-H, 5-H).
Synthesis of 4-Methylphenacylbromid structure (2a)
50.0 g (0.2 mol) 4-bromo acetophenone was solved in 100.0 ml pure diethyl ether in the 250.0 ml Erlenmeyer and then 12.5 ml (0.2 mol) bromine was added slowly to obtained solution and during the mixing, temperature of reaction container was kept to less than 20 °C. White aciform crystals were produced approximately 30 minutes after adding of bromine. Mixing was continued for 30 minutes later. Then content of Erlenmeyer was strained and dried. 48.0 g production by 83% yield was produced. Obtained precipitation was recrystallized by 95% ethanol for purification. Production was produced by 80-82 °C melting point. TLC chromatogram and comparison of its Rf by 4-methyl acetophenone Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (8:2 cyclo hexane–acetone) to give (48.0 g, 83%); A white solid; Mp: 80-82; 1H-NMR (400 MHz, CDCl3) δ 2.40 (s, 3H, CH3), 4.53 (s, 2H, CH2Br), 7.53-7.54 (d, 2H, 3-H, 5-H), 8.174-8.177 (J=9Hz, d, 2H, 2-H, 6-H).
Synthesis of 4-(4/-Bromophenyl)-2-mercaptothiazole structure (3a)
12.5 g (0.04 mol) 4-bromo phenacyl bromide was solved in 212.5 ml ethanol and 9.3 g (0.08 mol) ammonium di-thio carbamate was added to it and was refluxed for 3 hours. After ejecting of solvent by rotary, the yellow solid was formed that was refluxed for 15 minutes by 125.0 ml benzene. Than obtained solution was become cold and crystal precipitation was formed. Finally, the precipitation was strained and dried. 18 g production, 74% yield and 218 oC melting point was produced. TLC chromatogram and comparison of its Rf by 4-bromo phenacyl bromide Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (8:2 cyclo hexane–acetone) to give (18 g, 74%); A pale yellow; Mp: 216-218; IR (KBr): 3128-3050-1628-1401-1259-1180-746; 1H-NMR (400 MHz, CDCl3) δ 3.46 (s, 1H, SH Aliphatic), 7.37 (s, 1H, H-5), 7.61-7.63 (d, 2H, 2/-H, 6/-H), 7.67-7.69 (J=9Hz, d, 2H, 3-H/, 5-H/ ).
Synthesis of 4-(4/-methylphenyl)-2-mercaptothiazole structure (4a)
12.50 g (0.04 mol) 4-bromo phenacyl bromide was solved in 212.5 ml ethanol and 9.3 g (0.08 mol) ammonium di-thio carbamate was added to it and was refluxed for 3 hours. After ejecting of solvent by rotary, the yellow solid was formed that was refluxed for 15 minutes by 125.0 ml benzene. Than obtained solution was become cold and crystal precipitation was formed. Finally, the precipitation was strained and dried. 18.0 g production, 86% yield and 191-193 oC melting point was produced. TLC chromatogram and comparison of its Rf by 4-methyl phenacyl bromide Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (85:15 cyclo hexane–acetone) to give (18g, 86%); A yellow solid; Mp: 191-193; 1H-NMR (400 MHz, CDCl3) δ 2.40 (s, 3H, CH3), 3.16 (s, 1H, SH Aliphatic), 6.92 (s, 1H, H-5), 7.28-7.30 (J=9Hz, d, 2H, 3-H/،5-H/ ), 7.48-7.49 (d, 2H, 2/-H،6/-H).
Synthesis of 3-methyl-1-phenyl-2-pyrazolin-5-on structure (5a)
The production 5a was produced by three following method
Method number one
In the round bottom balloon, 100.0 ml ethanol was added to mixture of 33.3 g (0.2 mol) ethyl thio acetate and 27.7 g (0.2 mol) phenyl hydrazine and was refluxed in the oil container at 130 oC for 3 hours. After cooling, reaction mixture was put at the room temperature until crystals was formed and was filtered by Buchner funnel. Finally, it was recrystallized in ethanol.
Method number two
In the round bottom balloon, 14.7 g (0.1 mol) ethyl acetate and 10.9 g (0.1 mol) phenyl hydrazine was heated for 2 hours on steam bath distillation instrument was used for controlling of gases and doing of reaction at constant press. Then, reaction mixture was gotten cold and was mixed by adding of 20.0 ml cold di ethyl ether and produced solid was filtered and was washed by cold ether (50.0 ml) and dried. Finally, it was recrystallized in ethanol.
Method number three
Red oily mixture was produced from reaction of β-keto ester, ethyl acetate and phenyl hydrazine (1:1) respectively 14.7 g (0.1 mol) and 10.9 g (0.1 mol) in presence of concentrated sulfuric acid (4-5 drops) for 15 minutes. After 1 day, obtained solid was converted to powder by using of mortar. Powdered solid was washed by cold di ethyl ether. Then obtained powder was washed by 10% aqueous sodium carbonate (200.0 ml). 21.2 g pyrazoline was produce by 83% yield and 125-126 oC melting point. TLC chromatogram and comparison of its Rf by ethyl aceto acetate and phenyl hydrazine Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (8:2 cyclo hexane-acetone) to give (21.2 g, 83%); A yellow crystal; Mp: 125-126; 1H-NMR (400 MHz, CDCl3) δ 2.13 (s, 3H, CH3), 3.37 (s, 2H, Pyrazoline), 7.11 (t, 1H, 4-H/), 7.29-7.32 (J=9Hz, t, 2H, 3-H/, 5-H/ ), 7.71-7.73 (J=9Hz, d, 2H, 2/-H, 6/-H).
Synthesis of 3-propyl-1-phenyl-2-pyrazolin-5-on structure (6a)
In the round bottom balloon, 5.1 g (0.2 mol) ethyl butyryl acetate and 9.6 g (0.8 mol) phenyl hydrazine was heated on the steam bath for 2 hours. After cooling, 20.0 ml ether was added to reaction mixture and filtered. Then it was washed by ether and finally, it was recrystallized by ethanol. 10.0 g production was produced by 71% yield and 111 oC melting point. TLC chromatogram and comparison of its Rf by ethyl aceto acetate and phenyl hydrazine Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (75:15 n-hexane-acetone) to give (10 g, 71%); A yellow solid; Mp: 111-113; 1H-NMR (400 MHz, CDCl3) δ 1.07 (J=9Hz, t, 3H, CH3), 1.70 (J=9Hz, Sextet, 2H, CH2), 2.5 (J=9Hz, t, 2H, CH2), 3.46 (s, 2H, Pyrazoline), 7.22 (J=9Hz, t, 1H, 4-H/), 7.61-7.63 (J=9Hz, t, 2H, 3-H/،5-H/ ), 7.90-7.92 (J=9Hz, d, 2H, 2/-H،6/-H).
Synthesis of 1,3-diphenyl-2-pyrazolin-5-on structure (7a)
5.1 g (0.2 mol) ethyl benzoyl acetate and 9.6 g(0.8 mol) phenyl hydrazine was put in the round bottom balloon on the bath for 2 hours. After cooling, add 20.0 ml ether to reaction mixture and then filter the solid particles or wash them by ether. Finally, production was produced by 84% yield and then recrystallized by ethanol. TLC chromatogram and comparison of its Rf by ethyl benzoyl acetate Rf and phenyl hydrazine Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (25:75 n-hexane- acetone) to give (7.4 g , 84%); A yellow crystal; Mp: 112-115; 1H-NMR (400 MHz, CDCl3) δ 3.37 (s, 2H, CH2), 7.10-7.11 (J=9Hz, t, 2H, 4/-H), 7.69-7.77 (j=9Hz, d, 4H, 3-H, 5-H), 7.82-7.90 (j=9Hz, t, 4H, 2-H, 6-H).
Synthesis of 4-bromo-3-methyl-1-phenyl-2-pyrazolin-5-on structure (8a)
1.2 ml (0.4 mol) bromine (15% solution in acetic acid) was added to 4.0 g (0.2 mol) 3-methyl-1-phenyl-2-pyrazoline-5-on solution in 20.0 ml acetic acid during the mixing for 30 minutes at less than 10 °C temperature. At first, was washed by 25% acetic acid solution and then by 10% acetic acid solution. Aciform crystals were produced by 94% yield. Melting point after recrystallization in acetic acid was obtained 128-130°C. TLC chromatogram and comparison of its Rf by 3-methyl-1-phenyl-2 pyrazoline-5-on Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (85:15 cyclo hexane-acetone) to give (6.1 g, 94%); A white crystal; Mp: 128-130; 1H-NMR (400 MHz, CDCl3) δ 2.12 (s, 3H, CH3), 7.20-7.23 (d, 1H, 4-H), 7.42-7.45 (J=9Hz, d, 2H, 3-H, 5-H), 7.90-7.92 (t, 2H, 2-H, 6-H).
Synthesis of 4-bromo-3-propyl-1-phenyl-2-pyrazolin-5-on structure (9a)
1.5 ml (0.5 mol) bromine (15% solution in acetic acid) was added to 4.0 g (0.02 mol) 1-phenyl-3-propyl-2-pyrazoline-5-on solution in 20.0 ml acetic acid during the mixing for 30 minutes at less than 10 °C temperature. At first, obtained solid was washed by 25% acetic acid solution and then by 10% acetic acid solution. 4.7 g aciform crystals were produced by 85% yield and 128-130 °C melting point. TLC chromatogram and comparison of its Rf by 1-phenyl-3-propyl-2-pyrazoline-5-on Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (80:20 cyclo hexane-acetone) to give (4.7 g, 85%); A yellow crystal; Mp: 122-123; 1H-NMR (400 MHz, CDCl3) δ 0.88 (J=9Hz, t, 3H, CH3), 1.62 (m, 2H, CH2), 2.64 (J=9Hz, t, 2H, CH2), 5.82 (s, 1H, Pyrazoline), 7.30 (J=9Hz, t, 1H, 4-H), 7.40 (J=9Hz, d, 2H, 3-H, 5-H), 7.53 (J=9Hz, d, 2H, 2-H, 6-H).
Synthesis of 4-bromo-1,3-diphenyl-2-pyrazolin-5-on structure (10a)
1.5 ml (0.5 mol) bromine(15% solution in acetic acid) was added to 4.0 g (0.02 mol) 1,3-diphenyl-2-pyrazoline-5-on solution in 20.0 ml acetic acid during the mixing for 30 minutes at less than 10 °C temperature. At first, obtained solid was washed by 25% acetic acid solution and then by 10% acetic acid solution. 6.3 g white aciform crystals were produced by 84% yield. TLC chromatogram and comparison of its Rf by 1,3-diphenyl-2-pyrazoline-5-on Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (80:20 cyclo hexane–acetone) to give (6.3 g, 84%); A white crystal; Mp: 105-110; 1H-NMR (400 MHz, CDCl3) δ 7.40-7.55 (J=9Hz, t, 4H, 3-H, 5-H), 7.70 (t, 1H, 4-H), 8.10-8.20 (J=9Hz, d, 4H, 2-H, 6-H).
Synthesis of 2-(3-methyl-1-phenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-bromophenyl)thiazol structure (11a)
10.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001 mol) 2-mercapto-(4-bromo-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.0 g (0.002 mol) 4-bromo-3-methyl-1-phenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 7.5 g production by 84% yield was produced. It was recrystallized by absolute ethanol and finally white crystals were produced. TLC chromatogram and comparison of its Rf by 4-bromo-1,3-diphenyl-2-pyrazoline-5-on and 4-(4-bromo phenyl)-2-mercapto thiazole Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (80:20 cyclo hexane-acetone) to give (7.5 g, 84%); A white crystal; Mp: 128-135; 1H-NMR (400 MHz, CDCl3) δ 2.47 (s, 3H, CH3), 4.92 (J=9Hz, s, 1H, Pyrazole), 7.50-7.56 (J=9Hz, dd, 4H, 2//-H, 6//-H Aromatic), 7.55-7.77 (m, 5H, Phenyl), 7.60 (s, 1H, 5-H), 7.75-7.77 (J=9Hz, dd, 4H, 3//-H, 5//-H Aromatic).
Synthesis of 2-(3-propyl-1-phenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-bromophenyl)thiazol structure (12a)
9.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001mol) 2-mercapto-4-(4-bromo-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.1 g (0.002 mol) 4-bromo-3-propyl-1-phenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 9.8 g light yellow scaly crystals by 86% yield was produced. It was recrystallized by absolute ethanol. TLC chromatogram, melting point and comparison of its Rf by 4-bromo-1-phenyl-3-propyl-2-pyrazoline-5-on and 4-(4-bromo phenyl)-2-mercapto thiazole Rf was established Forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (80:20 cyclo hexane–acetone) to give (9.8 g, 86%); A yellow crystal; Mp: 140-145; 1H-NMR (400 MHz, DMSO) δ 1.07-1.09 (J=7Hz, t, 3H, CH3), 1.70-1.95 (J=7Hz, Sextet, 2H, Methylene), 2.83-3.00 (J=7Hz, t, 2H, CH2), 4.73 (J=9Hz, d, 1H, Pyrazoline), 7.42-7.72 (J=9Hz, m, 5H, 3/-H, 5/-H), 7.58 (J=9Hz, s, 1H, 5-H), 7.65-7.67 (J=9Hz, dd, 4H, 2//-H, 6//-H Aromatic), 7.70-7.77 (J=9Hz, dd, 4H, 3//-H, 5//-H Aromatic).
Synthesis of 2-(1,3-diphenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-bromophenyl)thiazol structure (13a)
10.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001mol) 2-mercapto-4-(4-bromo-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.1 g (0.002mol) 4-bromo-1,3-diphenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 8.6 g production by 91% yield was produced. It was recrystallized by absolute ethanol and finally yellow crystals were produced. TLC chromatogram and comparison of its Rf by 4-bromo-1,3-diphenyl-2-pyrazoline-5-on and 4-(4-bromo phenyl)-2-mercapto thiazole Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (25:75 n-hexane- acetone) to give (8.6 g, 95%); A pale yellow crystal; Mp: 132-135; 1H-NMR (400 MHz, CDCl3) δ 4.30 (s, 1H, Pyrazolone), 7.38 (s, 1H, 5-H), 5.47-5.48 (J=9HZ, dd, 4H, 2//-H, 6//-H Aromatic), 7.48-7.50 (J=9HZ, dd, 4H, 3//-H, 5//-H Aromatic), 7.38-7.86 (m, 10H, Aromatic), 7.58 (J=9Hz, d, 2H, 2/-H, 6/-H), 7.67(J=9Hz, d, 2H, 2//-H, 6//-H).
Synthesis of 2-(3-methyl-1-phenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-methylphenyl)thiazol structure (14a)
10.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001mol) 2-mercapto-4-(4-bromo-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.1 g (0.002mol) 4-bromo-3-methyl-1-phenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 7.5 g production by 84% yield was produced. It was recrystallized by absolute ethanol and finally gray scaly crystals were produced. TLC chromatogram and comparison of its Rf by 4-bromo-3-methyl-1-phenyl-2-pyrazoline-5-on and 4-(4-methyl phenyl)-2-mercapto thiazole Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (25:75 n-hexane- acetone) to give (7.8 g, 86%); A black crystal; Mp: 120-125; 1H-NMR (400 MHz, DMSO) δ 2.38 (s, 3H, CH3 Phenyl), 2.42 (s, 3H, CH3 Pyrazole), 4.42 (s, 1H, Pyrazolone), 7.35-7.85 (J=9Hz, m, Phenyl), 7.36 (s, 1H, 5-H), 7.52-7.60 (J=9Hz, dd, 4H, 2//-H, 6//-H Aromatic), 7.81-7.85 (J=9Hz, dd, 4H, 3//-H،5//-H Aromatic).
Synthesis of 2-(3-propyl-1-phenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-methylphenyl)thiazol structure (15a)
10.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001mol) 2-mercapto-4-(4-methyl-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.1 g (0.002mol) 4-bromo-1,3-diphenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 7.5 g production by 84% yield was produced. It was recrystallized by absolute ethanol and finally white scaly crystals were produced. TLC chromatogram and comparison of its Rf by 4-bromo-1,3-diphenyl-2-pyrazoline-5-on and 4-(4-methyl- phenyl)-2-mercapto thiazole Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (25:75 ethyl acetate- CCl4) to give (8.4 g, 86%); A white crystal; Mp: 123-125; 1H-NMR (400 MHz, DMSO) δ 1.05-1.08 (J=7Hz, t, 3H, CH3), 1.69-1.72 (J=7Hz, Sextet, 2H, CH2), 2.73-2.93 (J=7Hz, t, 2H, CH2), 2.78 (s, 3H, CH3), 4.57 (s, 1H, Pyrazoline), 4.58 (s, 1H, 5-H), 7.42-7.72 (m, 5H, Phenyl), 7.60-7.61 (J=7Hz, dd, 4H, 3//-H, 5//-H Aromatic), 7.61-7.62 (J=7Hz, dd, 4H, 2//-H, 6//-H Aromatic).
Synthesis of 2-(1,3-diphenyl-4,5-dihydro-5-oxopyrazol)-4-thio-4-(4-methylphenyl)thiazol (16a)
9.0 g sodium hydroxide (powdered) was added to solution contains of 5.0 g (0.001mol) 2-mercapto-4-(4-methyl-phenyl) thiazole in 250.0 ml absolute ethanol and was refluxed for 15 minutes. Then 6.0 g (0.002 mol) 4-bromo-3-methyl-1-phenyl-2-pyrazoline-5-on was added to obtained solution and reflux was continued for 45 minutes later. Then the solvent was separated by rotary. Some ice was added to residue solid and was washed by cool water. After drying, 8.6 g production by 91% yield was produced. It was recrystallized by absolute ethanol and finally brown scaly crystals were produced. TLC chromatogram and comparison of its Rf by 4-bromo-1,3-diphenyl-2-pyrazoline-5-on and 4-(4-methyl- phenyl)-2-mercapto thiazole Rf was established forming of production and its purity. The residue was purified by thin layer chromatography on silica gel (25:75 ethyl acetate-CCl4) to give (8.6 g, 91%); A brown crystal; Mp: 121-123; 1H-NMR (400 MHz, CDCl3) δ 2.42 (s, 3H, CH3), 4.32 (s, 1H, Pyrazlone), 7.38-7.72 (m, 10H, Phenyl), 7.39 (s, 1H, 5-H), 7.55-7.63 (J=9Hz, dd, 4H, 2//-H, 6//-H Aromatic), 7.70-7.72 (J=9HZ, dd, 4H, 3//-H, 5//-H Aromatic).
Result and Discussion
At first, a group of pyrazolines of was produced by using of beta ceto esters condensation reaction by hydrazine group, and then by using of aryl thiazoles bromination. Finally, intended production was produced from synthesis compounds. Some methods like NMR, elemental analysis and IR was used for identification of this compounds. TLC method, melting point and comparison of its Rf by other same compounds Rf was used for identification of purity degree. 4-bromo phenyl-2-mercapto thiazole was synthesized from reaction of ammonium di thio carbomate and N-bromo phenyl-5-pyrazolone synthesizes. From reaction of beta keto esters by phenyl hydrazine (see fig 1)
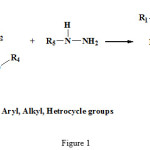 |
Figure 1: Synthetic route for the 2-pyrazolin-5-on derivatives. |
Pyrazolone and thiazole residues was joint to each other by sulfide bridge from condensation of 4-bromo-2-pyrazoline-5-on with 2-mercapto thiazoles by elimination of HBr (see fig 2-4)
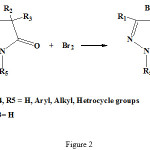 |
Figure 2: Synthetic route for the 4-Bromo-2-pyrazolin-5-on structure. |
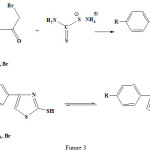 |
Figure 3: Synthetic route for the 4-phenyl-2mercapto thiazol structure. |
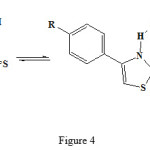 |
Figure 4: Different reaction of the 4-phenyl-2mercapto thiazol structure. |
Reactant, yield and reaction time had shown at table 1. However, color of aciform crystals had described at another column.
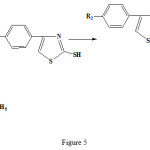 |
Figure 5: Synthetic route for the 4-(4-methylphenyl)-2-mercaptothiazole structure. |
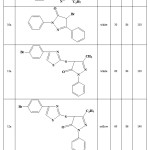 |
Table 1: Yields and reaction conditions of the synthesized compounds. |
Conclusion
Mechanisms and synthesis of 6 new structures of thiazoles family had shown that substitution of hydrogen atom by methyl group was caused to decreasing of pay and sigma electron density in carbon atom of the cycle. But there is small increasing in electron density when hydrogen atom is substituted by sulfur atom. As results had shown electron density of carbonic group decreased by substitution of chlorine group by NH2. Overall, obtained results had shown highest yield at all of mechanisms as compared with the same reactions and reaction rate is good and suitable.
Acknowledgement
The authors are indebted to Prof. Mohammad Rauof Darvish for their interest in this work and many helpful discussions. However this work was supported by Islamic Azad University Shahre-rey branch and Islamic Azad University Omidiyeh branch.
References
- A.Z. Amrita, G.P. Senthilkumar, Der Pharma Chemica, 3: 523 (2011).
- A. Hantzsch, H.J Weber, Ber Dtsch Chem Ges, 20: 3118 (1987).
- Sh, Jaiswal, A.P. Mishra, A. Srivastava, Research Journal of Pharmaceutical, Biological and Chemical Sciences, 3: 631 (2012).
- S.R. Pattan, N.S. Dighe1, S.A. Nirmal, A.N. Merekar, R.B Laware, H.V Shinde and D.S Musmade, Asian J. Research Chem, 2: 196 (2009).
- Ch.B. Guo, Zh.F. Cai, Z.R. Guo, Zh.Q. Feng, F.M.Chu, G.F.Cheng, Chinese Chemical Letters, 17: 325 (2006).
- H.I. El-Subbagh, A.H. Abadi, J. Lehmann, Arch. Pharm. Pharm. Med. Chem. 332: 137 (1999).
- D.R. Williams, A.O. Stewart, Tetrahedron letters, 37: 983 (1996).
- S.S. Kottawar, S.V. Goswami, P.B. Thorat, S.R. Bhusare, E-Journal of Chemistry, 8: 1859 (2011).
- B.S. Sathe, E. Jaychandran, G.M. Sreenivasa, V.A. Jagtap, E-Journal of Chemistry, 8: 830 (2011).
- W. Kasel, M. Dolezal, E. Sidoova, Z. Odlerova, J. Drsata, Chem Abstr, 110: 128063e (1989).
- U. Ronssel, Jpn Kokai Tokkyo Koho, Chem. Ast.r, 106: 156494G (1987).
- M. Fadayon, V.D. Kulkarni, A.S.H. Pakdaman, Asian J. Chem, 5: 282 (1993).
- P.S. Desai, K.R. Desai, J. Indian Chem. Soc, 71: 155 (1994).
- S.K. Srivastava, S. Srivastava, S.D. Srivastava, Indian J. Chem, 38: 183 (1999).
- J.J. Bhatt, B.R. Shah, P.B. Trivedi, N.K. Undavia, N.C. Desai, Indian J. Chem, 33: 189 (1994).
- B. Dash, P.K. Mahapatra, D. Panda, J.M. Patnaik, Indian Chem. Soc, 61: 1061 (1984).
- R.Yadav, S. Srivastava, S.K. Srivastava, S.D. Srivastava, Chemistry An IndianJournal, 1: 95 (2003).
- K.N. Venugopala, G.K. Rao, P.N.S. Pai, G.L. Ganesh, Asian Journal of Chemistry, 20: 1697 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









