Synthesis and Spectroscopic Studies of Zirconium(IV) Porphyrins with Acetylacetonate and Phenolates at Axial Positions
Gauri D. Bajju*, Sunil Kumar Anand and Sujata Kundan
Department of Chemistry, University of Jammu, New Campus, Baba Sahib Ambedkar Road, Jammu-180 006 (India).
Reaction of p-methoxy-meso-tetraphenylporphyrin(p-OCH3H2TPP) with Zirconium(IV) acetylacetonate(Zr(acac)4) and phenols at 200-220°C results in the formation of correspondimg axially ligated Zirconium(IV)-p-methoxy-mesotetraphenylporphyrin [Zr(p-OCH3TPP)(Y)(X)] [Y=acac and X=different phenolates]. The separation and isolation of these compounds have been achieved through chromatographic methods and characterized by 1HNMR, Electronic absorption spectra, IR spectra, elemental analysis and mass spectroscopy. Inseration of metal ion in the porphyrin ring is confirmed by absorption spectroscopy which shows normal two Q bands i.e., Q(0,0), Q(1,0) and one shoulder Q(2,0). The 1H NMR spectra of Zr(IV)porphyrins show upfield(shielded) compared to meso-tetraphenylporphyrin(H2TPP). IR spectra confirm the appearance of Zr-N at 457-502cm-1, Zr-O at 649-680cm-1 and also the incorporation of (acac)(C5H7O2) in which Zr-O is at 702-818cm-1. Mass spectra determine m/z ratio and percentage of each element is confirmed by elemental analysis.
KEYWORDS:Zirconium (IV) derivatives; Acetylacetonate; Axial positions
Download this article as:| Copy the following to cite this article: Bajju G. D, Anand S. K, Kundan S. Synthesis and Spectroscopic Studies of Zirconium(IV) Porphyrins with Acetylacetonate and Phenolates at Axial Positions. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Bajju G. D, Anand S. K, Kundan S. Synthesis and Spectroscopic Studies of Zirconium(IV) Porphyrins with Acetylacetonate and Phenolates at Axial Positions. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23989 |
Introduction
The involvement of porphyrins in many biological processes and the possibility to tailor their physical and chemical properties at the molecular level including very large dipole moments, polarizability, non-linear optical response, absorption spectrum, energy transfer and catalytic properties which make porphyrins and metalloporphyrins extremely versatile synthetic base materials for research projects in many disciplines of chemistry and physics, like electronic, opto-electronic, electrochemistry, catalysis and photophysics.Incorporation of porphyrins into zeolites has been explored as means to biomimetic oxidation catalysts (1). Another area of recent interest is to find application of porphyrin materials in the field of conducting polymers and ferroelectric materials (2). Furthermore, porphyrins and their structural analogues are used in broad technological applications such as electrophotographic photoreceptors, air deodorant, pigment preparations and photodynamic therapy (3) and in development of molecular electronic devices used in memory or logic applications (4). Over the past few years, the d0 transition-metal porphyrins containing Sc(III), Ti(IV), Zr(IV), Hf(IV), Nb(V), and Ta(V) have been synthesized and characterized (5-9). This has been mainly due to the use of these compounds in catalysis, photodynamic therapy of cancer cell (10), as materials with novel electrical properties (11) and as biomimetic model systems of primary processes of natural photosynthesis (12) and also due to their strong affinities for DNA and potential nuclease activity (13). The reactivity (14) and the previously reported synthetic methods for zirconium(IV) porphyrinates are generally carried out in refluxing solvents (benzonitrile, dimethoxyethane, or toluene) using the HfCl4 starting complex 80-95% yields of the M(Por)Cl2 complex. The metal ions in these complexes are oxophilic; thus, the preference for carboxylate or other oxygen-bearing anionic ligands. Zr(IV) has an electron configuration of [Kr]4f14, is closed shell metal ion and Because of the lack of functional groups on the porphyrin moieties, there is a paucity of supramolecular materials with designed, hierarchical structures (15-30) containing Zr(Por). The key entry to the organometallic zirconium porphyrin complexes would be Zr-(Porphyrin)Cl2, analogous to ZrCp2Cl2. The dichloride complex may be converted to organometallic σ-complexes such as dialkyl complexes by the reactions with alkyllithium or Grignard reagents. It may also form organometallic π-complexes by replacing the two chlorides with a cyclooctatetraenyl dianion or a dicarbollide dianion. Arnold and coworkers published (31) the synthesis and characterization of Zr(OEP)Cl2 and several organometallic complexes derived from it, including Zr(OEP)(CH2SiMe3)2 (31a) and Zr(OEP)(η5-C2B9H11) (31b). Also an extensive series of out of plane cis (OEP)ZrR2 dialkyls have been investigated due to their reactivity (32-36). These complexes undergo rapid Zr-C protonolysis, yielding new (OEP)ZrX2 (X¯=TfO¯,R’CO2¯Cl¯) and (OEP)Zr(R)+ derivatives, and also cleanly insert CO2 and acetone, yielding carboxylate and alkoxide complexes. Hydrogenolysis of (OEP)Zr(CH2SiMe3)2 yields hydride species which can be trapped by olefins yielding (OEP)Zr(CH2SiMe3)(CH2CH2R) and (OEP)Zr(CH2CH2R)2 (R=H,Me). This system catalytically hydrogenates ethylene, presumably via an insertion/Zr-C hydrogenolysis process. Recently, Inoue reported that (TPP)ZrX2 complexes (X¯=RCO2Cl¯)2 catalyze the carboalumination of terminal alkynes (37).
In this study, we report the synthesis of p-methoxy-meso-tetraphenylporphyrinatozirconium(IV)acetylacetonate with various phenols as axial ligands and spectroscopic parameters of these complexes.
Material and Methods
The Optical absorption spectra of the compounds were recorded on a Hitachi U-3400, lambda 35 UV- Vis. Spectrophotometer and Elico spectral treats UV-Vis. spectrophotometer using a pair of matched quartz cells of 10mm path length at an ambient temperature. The oscillator strength (ƒ) of the transitions in absorption spectra were calculated from the expression (38)
ƒ = 4.33 x 10-9 x ε x Δυ1/2
Where ε is the molar absorption coefficient in dm3 mol-1 cm-1 and Δυ1/2 is the full width at half maximum in cm-1.The 1H NMR spectra were recorded on an av 500 NMR Spectrometer in CDCl3 using tetramethylsilane (TMS) as internal standard.Porphyrin solutions (0.5 ml) of 10-2 to 10-3 M in CDCI3 were used for 1H NMR studies. The δ values reported are in ppm with number of protons involved followed by the position of protons.Infrared Spectra(IR-Spectra) were recorded on a PERKIN ELMER spectrometer at room temperature in KBr pellets. In Infrared spectroscopy, the detection of metal-nitrogen (M-N), metal-axial ligand (M-L) vibrations (39-42) and metalloporphyrins with different pure metal isotopes were studied over a large frequency range. The far-infrared region, which apparently provides valuable information on M-N vibrations, has been investigated. The elemental analysis of the precursor p-methoxy-meso-tetraphenylporphyrin and its Axially Ligated Zirconium(IV)acetylacetonate, with different phenolates as an axial ligand were performed on Elemental Analyser CHNS-932, LECO, USA at a temperature of about 1000oC using helium as carrier gas and oxygen for combustion.The MALDI Mass spectra were recorded on Bruker Daltonics Spectrophotometer. MALDI Data System in positive linear high power of detection at an accelerating voltage of 20 KV and laser power tuned depending on the sample and the spectra were recorded at room temperature and methanol as solvent.
Pyrrole (Fluka, Switzerland) was distilled over potassium hydroxide pellets under reduced pressure before use. Anisaldehyde(p-methoxybenzaldehyde) is procured from Aldrich, USA and used without further purification and Propionic acid used in the synthesis of tetraphenylporphyrin was obtained from Qualigens (India) and used as such. Silica gel (60-120 mesh) and silica gel (TLC grade, particle size = 75 μ procured from Merck, Germany) were used for column and thin layer chromatography respectively.Aluminium oxide (basic) purchased from Fluka, Switzerland and DDQ (2,3-dichloro-5,6-dicyano-p-benzoquinone) purchased from Merck, Germany were used as such.Zirconium(IV) acetylacetonate is purchased from Aldrich, USA and used without further purification.Anhydrous sodium sulphate (Na2SO4), potassium carbonate (K2CO3), sodium hydrogen carbonate (NaHCO3), sodium hydroxide (NaOH) and calcium chloride (CaCl2) procured from Ranbaxy Labs. Ltd. (India). The various phenols used were of AR grade (SISCO Research Laboratories Pvt. Ltd.) and used with further purification. The synthesis of the complexes of Zr(IV) was carried over a salt bath containing a mixture of NaNO3(53%), NaNO2(44%) and KNO3(7%). The salt melts at 142oC temperature. The temperature of the mixture was maintained between 230-240oC. The NaNO3, NaNO2 and KNO3 used for the preparation of salt bath were procured from Qualigens (India) and used as such.
p-methoxy-meso-tetraphenylporphyrin (p-OCH3H2TPP)
The preparation of p-OCH3H2TPP was carried out by condensation of pyrrole with anisaldehyde in refluxing propionic acid,p-OCH3H2TPP prepared was purified by column chromatography using CHCL3 as eluent. UV-vis(CHCL3): λmax(nm) : 424, 518.2, 557, 596, 650.9. 1HNMR (CDCL3) (δ,ppm): -2.10(s,2H,NH), 8.70(s,8H,β-pyrrole), 7.82(d,8H,Ho), 7.44(d,8H,Hm), 3.92(s,12H,Home). Anal.Calcd. for C48H38O4N4: C, 78.45; H, 5.21; N, 7.62. Found: C, 78.22; H, 5.22; N, 7.56.
p-methoxy-mesotetraphenylporphinatozirconium(IV)acetylacetonatophenoxide
[Zr(p-OCH3TPP)(acac)(Y)(X)]
Zr(acac)4(1.87×10-3moles),p-methoxy-mesotetraphenylporphyrin(3.74×10-4moles) and respective phenol(1.06×10-2 moles) were taken in a boiling tube covered with a funnel over the salt bath at about 200-220oC with constant stirring up to 25 minutes. After 25 minutes, the temperature was slightly raised and the given mixture was recharged with 1/3rd of initial amount of Zr(acac)4 and then again refluxed for 25 minutes. After cooling the reaction mixture, it was extracted with 2N boiling NaOH solution. The compound recovered after extraction was passed through Na2SO4. The solvent was recovered under reduced pressure and the reaction mixture was chromatographed through basic alumina using chloroform as an eluent, recrysatallised and characterized by UV-vis and 1HNMR spectra (scheme-1).
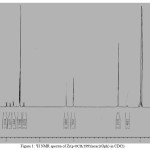 |
Figure 1: 1H NMR spectra of Zr(p-OCH3TPP)(acac)(Oph) in CDCl3 Click here to View figure |
Results and Discussion
1HNMR Spectroscopy
1H NMR was widely used as an analytical tool and the new structural insights that resulted were a major reason for the revival of interest in porphyrins chemistry. The 1H NMR spectra of the p-methoxy-meso-tetraphenylporphyrin and their Zr(IV) derivatives containing different phenolates as an axial ligand are highly characteristic and establish the structural integrity of these compounds in solution and data accumulated in (Table.1). The 1H NMR spectra of free-base porphyrins give three characteristic proton resonances (a) β-pyrrole protons (b) imino protons and (c) meso-aryl protons. The presence of subtituent in β-positions and peripheral phenyl groups alters these proton resonances. The integrated intensity of the resonances agrees well with the number of protons. The presence of electron-donating methoxy group at the para-position of the meso-phenyl ring causes a slight shielding effect of the β-pyrrole protons resulting in a slight upfield shift of meso-substituted porphyrins relative to simple meso-tetraphenylporphyrin(H2TPP).This shielding/deshielding is most likely a consequence of meso −C−C− dihedral angle much greater than 0o, thereby placing the b-pyrrole protons in a more shielded/deshielded environment produced by the anistropic effect of substituent ring current.The inner imino protons of the H2TPP resonate at -2.79 ppm, while those of p-OCH3H2TPP appear at -2.10 ppm. The meso-aryl protons of H2TPP resonate as a singlet at 8.19 ppm of ortho and 7.59 ppm of meta and para protons respectively and are shifted marginally depending upon the nature of the substituents attached as in case of p-OCH3H2TPP which has electron-donating methoxy groups, the resonance occurs at 7.82 ppm of ortho and 7.44 ppm of meta protons i.e, resonance is shifted upfield relative to H2TPP and meso-methoxy protons in p-OCH3H2TPP resonate as a singlet at 3.92 ppm respectively. Generally, the presence of axially ligated Zr(IV) metal in the porphyrin ring shifts the resonances to down-field accompanied by marginal changes in the pattern.One of the important feature of axially ligated Zr(IV) derivatives of porphyrins is that the metal is almost certainly out of the plane of the porphyrin ring which have earlier been reviewed in literature, responsible for the production of asymmetric environment above and below the plane of the macrocycle which ultimately account for the pronounced non-equivalence of the ortho protons of the phenyl rings. In axially ligated zirconium compounds of p-OCH3TPP, a slight shielding (upfield) of protons is observed with respect to H2TPP (Table 2). The 1H NMR of Zr(p-OCH3TPP)(acac)(p−NO2PhO),indicates that the β-pyrrole protons resonate as singlet at 9.18 ppm, the meso-aryl ortho protons resonate as duplet at 8.33 ppm and 8.07 ppm, the meso-aryl meta protons resonate as duplet at 7.81 ppm, the meso-methoxy protons resonate as singlet 4.24 ppm, the acac protons resonate as two singlets at 1.76 ppm and 4.51 ppm, the ortho protons of para nitro phenolate resonate as duplet at 7.13 ppm and the meta-protons of para nitro phenolate resonate as duplet at 7.32 ppm respectively which is downfield with respect to Zr(p-OCH3TPP)(acac)(Oph) (Figure. 1). In case of Zr(p-OCH3TPP)(acac)(p-NH2phO, the β-pyrrole protons resonate as singlet at 8.32 ppm, the meso-aryl ortho protons resonate as duplet at 7.32 ppm and 7.19 ppm the meso-aryl meta protons resonate as multiplet at 7.02-7.10 ppm, the meso-methoxy protons resonate as singlet 4.01 ppm, the acac protons resonate as two singlets at 1.47 ppm and 3.71 ppm, the amino protons of para amino phenolate resonate as singlet at 4.65 ppm, the ortho protons of para amino phenolate resonate as duplet at 6.74 ppm and the meta-protons of para amino phenolate resonate as duplet at 6.58 ppm respectively which is upfield. Thus, for Zr(p-OCH3TPP)(acac)(p-NO2phO),the resonance of protons occur slightly downfield with respect to Zr(p-OCH3TPP)(acac)(Oph) because of presence of −NO2 at para-position of phenolate which are electron withdrawing groups and responsible for deshielding and for Zr(p-OCH3TPP)(acac)(p-NH2phO), the resonance of protons occur slightly upfield with respect to Zr(p-OCH3TPP)(acac)(phO) because of presence of −NH2 at para-position of phenolate which are electron releasing groups and are responsible for shielding.
Table 1 : 1H NMR data of free-base porphyrin (p-OCH3H2TPP) and Zr(p-OCH3TPP)(Y)(X) (Y = acac and X = different phenolates as an axial ligand) in CDCl3 at 298 K.
|
Porphyrins |
b-Pyrrole Protons |
Imino Protons |
Meso-aryl Protons |
Other Protons |
|
|
acac protons |
Phenolate protons |
||||
| p-OCH3 H2TPP(C48H38O4N4) | 8.70(s) | -2.10(s) | 7.82(d,8H,Ho)7.44(d,8H,Hm)
3.92(s,12H,HOMe) |
– | – |
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] | 8.83(s) | – | 8.02(d,4H,Ho)7.77(d,4H,Ho)
7.57(d,8H,Hm) 4.13(s,12H,HOMe) |
1.69(s,6H,HMe)4.44(s,H,HCH) | 6.98(d,2H,Ho)7.05-7.19(m,3H,Hm,p) |
| Zr(p-OCH3TPP)(acac)(p-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] | 9.15(s) | – | 8.27(d,4H,Ho)8.02(d,4H,Ho)
7.76(d,8H,Hm) 4.19(s,12H,HOMe) |
1.72(s,6H,HMe)4.47(s,H,HCH) | 7.04(d,2H,Ho)7.21(d,2H,Hm) |
| Zr(p-OCH3TPP)(acac)(p-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] | 9.18(s) | – | 8.33(d,4H,Ho)8.07(d,4H,Ho)
7.81(d,8H,Hm) 4.24(s,12H,HOMe) |
1.76(s,6H,HMe)4.51(s,H,HCH) | 7.13(d,2H,Ho)7.32(d,2H,Hm) |
| Zr(p-OCH3TPP)(acac)(p-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] | 8.32(s) | – | 7.32(d,4H,Ho)7.19(d,4H,Ho)
7.02-7.10(d,8H,Hm) 4.01(s,12H,HOMe) |
1.47(s,6H,HMe)3.71(s,H,HCH) | 6.74(d,2H,Ho)6.58(d,2H,Hm)
4.65(s,2H, HNH2) |
| Zr(p-OCH3TPP)(acac)(p-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] | 8.36(s) | – | 7.42(d,4H,Ho)7.37(d,4H,Ho)
7.08-7.19(d,8H,Hm) 4.06(s,12H,HOMe) |
1.51(s,6H,HMe)3.76(s,H,HCH) | 6.89(d,4H,Ho,m)6.82(d,2H,Hm)
3.37(s,3H,HOMe) |
Table 2 : Optical Absorption data of Zr(p-OCH3TPP)(Y)(X), (Y = acac and X = different phenolates as an axial ligand in CHCl3 showing lmax together with log e and n1/2.
| Compounds |
B-bands lmax(nm), (log e)(M-1 cm-1) n1/2(cm-1) |
Q-bands lmax(nm), (log e) (M-1 cm-1) n1/2(cm-1) |
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] | 414.4, (5.098), 1320.5 | 501.3, (4.027)537.3, (4.826)
581.6, (5.040), 1075.3 |
| Zr(p-OCH3TPP)(acac)(p-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] | 415.6, (5.129), 1349.2 | 502.4, (4.219)538.8, (4.839)
583.3, (5.067), 1094.4 |
| Zr(p-OCH3TPP)(acac)(o-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] | 415.5, (5.121), 1350.4 | 502.2, (4.216)538.5, (4.836)
583.1, (5.060), 1096.6 |
| Zr(p-OCH3TPP)(acac)(m-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] | 415.1, (5.116), 1336.6 | 501.9, (4.211)538.1, (4.835)
582.6, (5.054), 1089.4 |
| Zr(p-OCH3TPP)(acac)(p-CH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O)] | 415.9, (5.134), 1347.6 | 502.6, (4.223)539.2, (4.844)
583.8, (5.069), 1091.9 |
| Zr(p-OCH3TPP)(acac)(o-CH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O)] | 415.7, (5.127), 1349.8 | 502.7, (4.221)538.8, (4.837)
583.7, (5.064), 1096.3 |
| Zr(p-OCH3TPP)(acac)(p-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] | 413.4, (5.080), 1285.2 | 500.2, (4.197)536.2, (4.817)
580.7, (5.024), 1045.3 |
| Zr(p-OCH3TPP)(acac)(o-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] | 413.2, (5.077), 1282.8 | 500.1, (4.195)536.1, (4.816)
580.5, (5.023), 1043.1 |
| Zr(p-OCH3TPP)(acac)(m-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] | 413.7, (5.085), 1291.4 | 500.6, (4.199)536.8, (4.819)
580.9, (5.029), 1049.5 |
| Zr(p-OCH3TPP)(acac)(p-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] | 414.6, (5.095), 1303.2 | 501.5, (4.205)537.6, (4.825)
582.1, (5.037), 1060.3 |
| Zr(p-OCH3TPP)(acac)(o-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] | 414.2, (5.093), 1299.8 | 501.1, (4.203)537.2, (4.823)
581.9, (5.035), 1058.1 |
| Zr(p-OCH3TPP)(acac)( p-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] | 416.8, (5.149), 1368.9 | 503.4, (4.234)540.5, (4.857)
584.9, (5.086), 1102.1 |
| Zr(p-OCH3TPP)(acac)( o-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] | 416.6, (5.143), 1364.5 | 503.1, (4.229)539.9, (4.855)
584.5, (5.079), 1099.5 |
| Zr(p-OCH3TPP)(acac)( m-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] | 416.1, (5.139), 1361.7 | 502.7, (4.227)539.6, (4.849)
584.3, (5.077), 1095.9 |
| Zr(p-OCH3TPP)(acac)( 2,4-dichlorophO)[Zr(C48H36O4N4)(C5H7O2)(C6H3OCl2)] | 413.6, (5.088), 1268.7 | 499.8, (4.206)535.8, (4.821)
580.3, (5.030), 1035.7 |
| Zr(p-OCH3TPP)(acac)( a-naptholate)[Zr(C48H36O4N4)(C5H7O2)(C10H7O)] | 414.1, (5.096), 1306.8 | 501.0, (4.204)537.1, (4.822)
581.3, (5.038), 1061.2 |
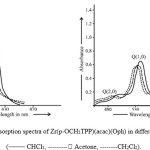 |
Figure 2 : Optical absorption spectra of Zr(p-OCH3TPP)(acac)(Oph) in different solvents (––––– CHCl3, ——— Acetone, ——–CH2Cl2). |
Absorption Spectroscopy
The optical absorption spectrum is an important spectral phenomenon to distinguish between the free base porphyrins and their metalloderivatives based on profound theoretical analysis of the experimental and quantum-chemical data of the previously mentioned monograph (43). According to this interpretation the absorption spectra of porphyrins do not exhibit bands of n→π* transitions because of the symmetry of the n orbitals and antisymmetry of the n orbitals with respect to the plane of the porphyrin molecule. All bands are of π→π* origin. The Soret band is due to an electronic 1A1g → 1E′u transition to the highest energy vacant π* orbital. In substituted porphyrins E′u is split into two states B′2u and B′3u which are close in energy, as a result of the reduced symmetry of the π-electron cloud. The electronic transitions to state E′u (or B′2u and B′3u) are allowed, therefore the intensity of the Soret band is always very high (ε ≈105). Bands I and III of the visible region belong to quasi-forbidden electronic transitions. The reasons for transitions 1A1g → B′3u and 1A1g → B′2u being allowed, which is responsible for the appearance of band I and III. Bands II and IV are of vibrational origin, that is they are vibrational satellites of bands II and III, respectively. A factor that must be considered in the interpretation of the spectra in the visible and ultraviolet region is that the phenyl and substituted phenyl rings cannot be coplanar with the porphine nucleus. Examination of molecular models indicates that the four benzene rings have partial rotations that cannot bring them with in 60o of being coplanar with the resonating porphine system.Since the average angle of the attached rings is probably considerable greater than this. It is apparent that resonance interactions between two aromatic systems must be greatly reduced from what would be expected if they were coplanar. Hence, the bathochromic shifts and spectral intensifications observed are considerably less than what would be expected in simpler aromatic systems with greater freedom of rotation.
Generally, the absorption spectra of group IV B porphyrins are quite “normal” in that they exhibit the expected N, B and Q bands (44). The N bands are less intense and appear at or below 350 nm. The optical absorption spectral data of Zr(IV) metal derivatives containing acetylacetonate (acac) and different phenolates as an axial ligand in chloroform is shown in the (Table. 2). It is observed from the tables that the Zr(IV) metal derivatives of
(p-OCH3TPP) with phenolates as an axial ligand show hypso-chromic shift (blue-shift) and variation in intensities of absorption bands when compared to their respective free base porphyrins (H2TPP), due to incorporation of the metal ion alongwith phenolate in the porphyrin rings. When a comparative study of optical absorption spectral data of Zr(p-OCH3TPP)(Y)(X), (Y = acac and X = different phenolates as an axial ligand) in chloroform is done with respect to Zr(H2TPP)(Y)(X), a slight bathochromic shift (Red shift) i.e., to longer wavelength is observed because of the presence –OCH3group at the para position of the meso-phenyl rings of porphyrin moiety. The optical absorption spectra of Zr(IV) metal derivatives of different porphyrins with different phenolates as an axial ligand show one soret band i.e. B(0, 0), two Q bands i.e., Q(0, 0), Q(1, 0) and one shoulder Q(2, 0). The order of absorbance of B and Q bands of axially ligated Zr(IV) metal derivatives of different porphyrins is B(0, 0) > Q(0, 0) > Q(1, 0) > Q(2, 0). When a comparative study is done among the axially ligated Zr(IV) porphyrins, with different phenolates attached to Zr(IV) metal atom , those having electron donating groups in phenolates have slightly red shifted B and Q bands while those having electron withdrawing groups in phenolates have blue shifted B and Q bands. When the optical absorption spectra of the compounds of Zr(p-OCH3TPP)(Y)(Y) is recorded in different solvents (Table. 3) and spectra of Zr(p-OCH3TPP)(acac)(Oph) is displayed in (Figure. 2), which shows only a marginal change in λmax values, absorption coefficient (ε) and oscillator strength (ƒ) values is observed. The data also reveal that a change in polarity of the solvent result in slight change in the position of transitions but there is a significant increase in “Fwhm” (υ1/2) and ‘ƒ’ values of transitions by increasing the polarity of the solvent. The magnitude of change of the ‘ƒ’ value in axially ligated Zr(IV) metal derivatives of different porphyrins reveal the relative strength of π→π* interactions. It is found that with the increase in polarity of the solvents, B and Q-bands in axially ligated Zr(IV) metal derivatives with different porphyrins show a red shift with progressive broadening of bands indicating that the magnitude of red shift of B and Q bands depends on the nature of the solvent used.
Table 3 : Optical Absorption data of Zr(p-OCH3TPP) (Y)(X), (Y = acac and X = different phenolates as an axial ligand) in different solvents.
| Compounds |
Solvent |
lmax(nm), (log e) (M-1cm-1) |
n1/2(cm-1) |
Q(0, 0) f |
||||
|
B(0,0) |
Q(2,0) |
Q(1,0) |
Q(0,0) |
B(0,0) |
Q(0,0) |
|||
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] |
CHCl3 CH2Cl2 Acetone |
414.4 5.098 409.7 5.066 411.8 5.077 |
501.8 4.206 496.4 4.177 500.2 4.200 |
537.3 4.826 531.7 4.773 535.5 4.786 |
581.6 5.040 575.9 4.986 578.8 4.993 |
1320.5 1262.6 1296.3 |
1075.3 1038.6 1057.3 |
0.255263 0.217724 0.225246 |
| Zr(p-OCH3TPP)(acac)(o-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] |
CHCl3 CH2Cl2 Acetone |
416.6 5.143 414.2 5.102 415.4 5.114 |
503.1 4.228 500.3 4.190 501.5 4.209 |
539.9 4.855 536.4 4.803 537.9 4.820 |
584.5 5.079 580.7 5.019 582.4 5.028 |
1364.5 1319.5 1344.8 |
1099.5 1060.3 1081.6 |
0.285531 0.239821 0.249761 |
| Zr(p-OCH3TPP)(acac)(m-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] |
CHCl3 CH2Cl2 Acetone |
413.2 5.085 408.1 5.039 409.8 5.056 |
500.1 4.199 494.9 4.162 498.2 4.181 |
536.1 4.819 531.2 4.765 533.4 4.780 |
580.5 5.029 574.8 4.963 577.6 4.985 |
1291.4 1232.1 1270.3 |
1049.5 1007.2 1030.4 |
0.242907 0.200250 0.215508 |
 |
Figure 3: IR Spectra of Zr(p-OCH3TPP)(acac)(Oph) Click here to View figure |
Infra Red Spectroscopy
The IR spectra of free base porphyrins(H2TPP) and its axially ligated Zr(IV) metal derivatives containing -OCH3 group have strong absorption bands of ν(C-H), ν(C-O-C)sym and ν(C-O-C)asym at (2810 – 2955cm-1), (1000 – 1050cm-1) and (1200–1300cm-1) In p-OCH3H2TPPP, ν(N-H) vibrates at 3446.5cm-1, aromatic ν(C-H) at 2963.3cm-1, ν(C-H) at 1350.2 cm-1,ν(C=C) at 1567.8 cm-1 and additional vibrations due to presence -OCH3 group at p-position of meso-phenyl ring also occurs in which ν(C-H) vibrates at 2906.8cm-1, ν(C-O-C)sym at 1019.4cm-1 and ν(C-O-C)asym at 1259.6cm-1 respectively. The IR absorption spectra of p-OCH3TPPand their corresponding axially ligated Zr(IV) acetylacetonate (acac) and different phenolates as an axial ligand is shown in the (Table. 4). The metallation of porphyrin is confirmed by the appearance of Zr–N band (45) in the range of 457–502cm-1. The incorporation of various phenolates in Zr(p-OCH3H2TPP)(Y)(X), (Y = acetylacetonate (C5H7O2) and X = different phenolates as an axial ligand) is confirmed by the appearance of Zr–O vibrational frequencies in the range of 649–680cm-1 and acetylacetonate(acac) is confirmed by the appearance of C=O vibrational frequencies in the range of 1617–1641cm-1 and Zr–O in the range of 702–818.5cm-1 respectively. In case of IR spectra Zr(p-OCH3H2TPP)(acac)(Oph) (Figure.3), aromatic ν(C-H) vibrates at 2952.5cm-1,ν(C-N) at 350.3cm-1, ν(C=C) at 1589.3cm-1, ν(Zr-N) at 495.1cm-1, ν(Zr-O) of phenolate at 664.2cm-1,ν(Zr-O) of acac at 702.0cm-1 and 802.4 cm-1,ν(C=O) of acac at 1630.5cm-1 and methoxy group vibration appear for ν(C-H), at 2825.1cm-1 for ν(C-H), at 2825.1cm-1,ν(C-O-C)sym at 1023.1cm-1 and ν(C-O-C)asym at 1261.5cm-1.
Table 4 : Main Vibrational frequencies corresponding to the various groups in Zr(p-OCH3TPP)(Y)(X), (Y = acac and X = different phenolates as an axial ligand).
| Porphyrin | v(N-H)(cm-1) | n(C-H)(cm-1) | n(C-N)(cm-1) | n(C=C)(cm-1) | n(Zr-N)(cm-1) | n(Zr-O) of
phenolate (cm-1) |
n(Zr-O) of acac
(cm-1) |
n(CH3)(cm-1) | n(OCH3)(cm-1) | n(NH2)(cm-1) | n(NO2)(cm-1) | n(C-Cl) (cm-1) | n(C=O) of acac
(cm-1) |
| p-OCH3H2TPP[C48H38O4N4] | 3446.5 | 2963.3 | 1350.2 | 1567.8 | – | – | – | – | n(C– H)=2906.8n(C–O–C)sym=1019.4
n(C–O–C)asym=1259.6 |
– | – | – | – |
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] | – | 2952.5 | 1350.3 | 1589.3 | 495.1 | 664.2 | 702.0802.4 | – | n(C– H)=2825.7n(C–O– C)sym=1023.1
n(C–O–C)asym=1261.5 |
– | – | – | 1630.5 |
| Zr(p-OCH3TPP)(acac)(p-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] | – | 2949.1 | 1349.8 | 1579.3 | 462.2 | 651.4 | 702.5802.6 | – | n(C– H)=2842.3n(C–O–C)sym=1022.6
n(C–O–C)asym=1260.9 |
– | – | – | 1630.9 |
| Zr(p-OCH3TPP)(acac)(a-naptholate)[Zr(C48H36O4N4)(C5H7O2)(C10H7O)] | – | 2949.3 | 1350.0 | 1587.4 | 471.6 | 665.1 | 702.3802.7 | – | n(C– H)=2824.2n(C–O–C)sym=1023.0
n(C–O–C)asym=1261.4 |
– | – | – | 1630.7 |
| Zr(p-OCH3TPP)(acac)(p-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] | – | 2958.3 | 1350.7 | 1590.4 | 470.6 | 663.2 | 703.2802.9 | – | n(C– H)=2953.6n(C–O–C)sym=1023.4
n(C–O–C)asym=1261.8 |
– | – | 780.9 | 1633.1 |
| Zr(p-OCH3TPP)(acac)(p-CH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O)] | – | 2942.8 | 1348.4 | 1573.2 | 466.3 | 649.1 | 702.3802.5 | 2890.2 | n(C– H)=2841.2n(C–O–C)sym=1021.8
n(C–O–C)asym=1260.3 |
– | – | – | 1629.8 |
| Zr(p-OCH3TPP)(acac)(2,4-dichlorophO)[Zr(C48H36O4N4)(C5H7O2)(C6H3OCl2)] | – | 2961.6 | 1351.9 | 1590.9 | 476.3 | 665.9 | 703.9804.1 | – | n(C– H)=2954.2n(C–O–C)sym=1023.6
n(C–O–C)asym=1262.1 |
– | – | 785.1 | 1635.4 |
| Zr(p-OCH3TPP)(acac)(p-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6HON)] | – | 2963.7 | 1350.4 | 1592.2 | 457.4 | 654.1 | 702.9802.8 | – | n(C– H)=2849.4n(C–O–C)sym=1023.0
n(C–O–C)asym=1261.5 |
n(NH2)sym= 3289.3
n(NH2)asym = 3367.1 |
– | – | 1631.6 |
| Zr(p-OCH3TPP)(acac)(p-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] | – | 2960.4 | 1351.5 | 1590.8 | 479.4 | 665.6 | 704.3804.5 | – | n(C– H)=2955.3n(C–O–C)sym=1023.8
n(C–O–C)asym=1262.7 |
– | n(NO2)sym= 1341.3
n(NO2)asym = 1540.9 |
– | 1635.2 |
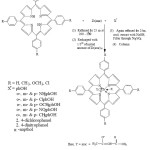 |
Scheme 1: General Scheme for the preparation of axially ligated Zirconium (IV) porphyrins with different phenolates as an axial ligands Click here to View scheme |
Elemental Analysis
The purity of Zr(IV) metal derivative of p-methoxy-meso-tetraphenylporphyrin containing different phenolates as axial ligand are also characterized by their elemental anylasis and data is accumulated in (Table. 5).
Table 5 : Elemental Analytical data of Zr(p-OCH3TPP)(acac)(Y)(X) (Y = acac and X = different phenolates as an axial ligand) along with their calculated values.
| Compounds |
Calculated Percentage |
Found Percentage |
||||
|
C |
H |
N |
C |
H |
N |
|
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] |
69.72 |
4.76 |
5.51 |
69.59 |
4.74 |
5.46 |
| Zr(p-OCH3TPP)(acac)( p-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] |
64.05 |
4.79 |
6.79 |
63.94 |
4.76 |
6.72 |
| Zr(p-OCH3TPP)(acac)(o-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] |
62.87 |
4.51 |
5.33 |
62.78 |
4.52 |
5.29 |
| Zr(p-OCH3TPP)(acac)(p-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] |
68.88 |
4.82 |
5.36 |
68.73 |
4.80 |
5.31 |
| Zr(p-OCH3TPP)(acac)(a-naptholate)[Zr(C48H36O4N4)(C5H7O2)(C10H7O)] |
70.96 |
4.73 |
5.25 |
70.84 |
4.67 |
5.21 |
| Zr(p-OCH3TPP)(acac)(p-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] |
66.77 |
4.46 |
6.60 |
66.64 |
4.45 |
6.55 |
| Zr(p-OCH3TPP)(acac)(2,4-dichlorophO)[Zr(C48H36O4N4)(C5H7O2)(C6H3OCl2)] |
65.30 |
4.27 |
5.16 |
65.19 |
4.19 |
5.12 |
| Zr(p-OCH3TPP)(acac)(p-CH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O)] |
69.95 |
4.89 |
5.44 |
69.72 |
4.63 |
5.38 |
Mass Spectroscopy
Mass spectroscopy is the most accurate method employed for determining the molecular mass of the axially ligated Zr(IV) metal derivative containing acetylacetonate and different phenolates as an axial ligand and their data is accumulated in (Table. 6) and spectra of some representative compound are displayed in the (Figure.4(a) and 4(b)).
Table 6 : Mass Data for Zr(p-OCH3TPP)(Y)(X), (Y = acac and X = different phenolates as an axial ligand).
| Compounds |
m/z ratio |
|
|
Observed |
Calculated |
|
| Zr(p-OCH3TPP)(acac)(Oph)[Zr(C48H36O4N4)(C5H7O2)(C6H5O)] |
1015.7 |
1016.28 |
| Zr(p-OCH3TPP)(acac)(p-NH2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H6ON)] |
1030.9 |
1031.29 |
| Zr(p-OCH3TPP)(acac)(p-ClphO)[Zr(C48H36O4N4)(C5H7O2)(C6H4OCl)] |
1051.2 |
1050.72 |
| Zr(p-OCH3TPP)(acac)(p-OCH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O2)] |
1046.5 |
1046.30 |
| Zr(p-OCH3TPP)(acac)(a-naptholate)[Zr(C48H36O4N4)(C5H7O2)(C10H7O)] |
1065.4 |
1066.34 |
| Zr(p-OCH3TPP)(acac)(p-NO2phO)[Zr(C48H36O4N4)(C5H7O2)(C6H4O3N)] |
1060.6 |
1061.27 |
| Zr(p-OCH3TPP)(acac)(2,4-dichlorophO)[Zr(C48H36O4N4)(C5H7O2)(C6H3OCl2)] |
1085.8 |
1085.17 |
| Zr(p-OCH3TPP)(acac)(p-CH3phO)[Zr(C48H36O4N4)(C5H7O2)(C7H7O)] |
1029.2 |
1030.31 |
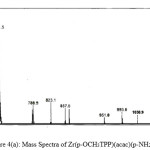 |
Figure 4(a): Mass Spectra of Zr(p-OCH3TPP)(acac)(p-NH2phO) Click here to View figure |
 |
Figure 4(b): Mass Spectra of Zr(p-OCH3TPP)(acac)(p-ClphO) Click here to View figure |
Conclusion
On the basis of above studies,it is found that the environment around Zr(IV) metal in their axially ligated porphyrins could be depicted as shown in the scheme.2.
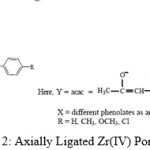 |
Scheme 2: Axially Ligated Zr(IV) Porphyrins |
References
- (a) Bediqui F., Coord. Chem. Rev., 144, 39(1995).(b) Liu C. J., Li S. G., Pang W. Q. and Chen C. M., Chem. Commun., 65(1995).
- (a) Collman J. P., McDevitt J. T., Yee G. T., Leidner C. R., McCullough, L. G., Little, W. A. and Torrance, J. B., Proc. Natl. Acad. Sci. USA., 83, 4581(1986). (b) Chen C. T. and Suslick K. S., Coord. Chem. Rev., 128, 293(1993). (c) Segawa H., Kunimoto K., Susumu K., Taniguchi M. and Shimidzu T., J. Am. Chem. Soc., 116, 11193(1994).
- Doughtery J. J., Photochem. Photobiology., 45,879(1987).
- (a) Bindtead R.A., Crossley M. J.and Hush N. S., Inorg. Chem., 30, 1259(1991). (b) Bonfantini E. E. and Officer D. L., J. Chem. Soc. Chem. Commun., 1445(1994).
- Buchler J. W., Elkelmann G., Puppe L., Rohbock K., Schneehage H. H.and Weck D., Justus Liebigs Ann. Chem., 745, 135(1971).
- Buchler J. W.and Rohbock K., Inorg. Nucl. Chem. Lett., 8, 1073(1972).
- Buchler J. W., Puppe L., Rohbock K.and Schneehage H. H., Ann. N.Y. Acad. Scl., 206, 116(1973).
- (a) Tsutsui M., Velapoldi R. A., Suzuki K., Vohwinkel F., Ichakawa M. and Koyano T., J. Am. Chem. Soc., 91, 6262(1969). (b) Tsutsui M., Velapoldi R. A., Suzuki K.and Koyano T., Angew. Chem., 80, 914(1968).
- Fuhrhop J.-H., Tetrahedron Lett., 3205(1969).
- (a) Sternberg E. D.and Dolpin D., Tetrahedron., 54(1998). (b) Bonnett R., Chem. Soc. Rev., 24, 19(1995).
- (a) Wagner R. W.and Lindsey J. S., J. Am. Chem. Soc., 119, 9759( 1994). (b) Osuka A.and Shimidzu H., Angew. Chem., Int. Ed. Engl., 36, 135(1997).
- (a) Kurreck H.and Huber M., Angew. Chem. Int. Ed. Engl., 34, 849(1995). (b) Wasielewski M. R., Chem. Rev., 92, 435(1992).
- (a) Fiel R. B.and Biomel J., Structure and Dynamics., 6, 1259( 1989). (b) Bromley S. D., Ward B. W.and Dabrowiak J. C., Nucleic Acid Res., 14, 9133(1986). (c) Pasternack R. F. and Gibbs E. J., ACS Symp. Ser., 402, 59(1989). (d) Sari M. A., Battioni J. P., Dupre D., Mansuy D. and Le Peg J. B., Biochemistry., 29, 4205(1990).
- Brand H.and Arnold J., Organometallics., 12, 3655(1993).
- Drain C. M.and Lehn J.-M., Chem. Commun., 2313(1994).
- Drain C. M., Nifiatis F., Vasenko A.and Batteas J. D., Angew. Chew., Int. Ed., 37, 2344(1998).
- Drain C. M., Proc. Natl. Acad. Sci., U. S. A., 99, 5178( 2002).
- Drain C. M., Batteas J. D., Flynn G. W., Milic T., Chi N., Yablon D. G. and Sommers H., Proc. Natl. Acad. Sci., U.S.A, 99, 6498(2002).
- Drain C.M., Chen X. and Nalwa H.S., Ed. Encyclopedia of Nanoscience & Nanotechnology, Vol.IX, American Scientific Press, New York, 593(2004).
- Cheng K. F.,Thai, N. A., Teague L. C., Grohmann K.and Drain C. M., Chem. Commun., 4678(2005).
- Drain C. M.,Bazzan G., Milic, T., Vinodu, M.and Goeltz, J. C., Isr. J. Chem., 45, 255(2005).
- Drain, C. M., Goldberg, I., Goldberg, I.and Falber, A., Top. Curr. Chem., 245, 55(2005).
- Diskin-Posner Y., Dahal, S.and Goldberg, I., Angew. Chem. Int. Ed., 39, 1288(2000).
- Goldberg I., Chem. –Eur. J., 6, 3863(2000).
- Diskin-Posner., Patra G. K.and Goldberg. I., Eur. J. Inorg. Chem., 2001, 2515(2001).
- Goldberg I., Cryst. Eng. Commun., 4, 109(2002).
- Diskin-Posner Y., Patra, G. K. and Goldberg I.,Cryst. Eng. Commun., 4, 296(2002).
- Shmilovits M., Diskin-Posner Y., Vinodu M.and Goldberg I., Cryst. Growth Des., 3, 855(2003).
- Goldberg I., Chem. Commun., 1243(2005).
- Lee S. J.and Hupp J. T., Coord. Chem. Rev., 250, 1710(2006).
- (a) Brand H.and Arnold J. J.Am.Chem.Soc., 114, 2266(1992). (b) Arnold J., Johnson S. E., Knobler C. B.and Hawthorne M. F., Ibid., 114, 3996(1992).
- Brand., Holger., Arnold J., J. Am. Chem. Soc., 144, 2266(1992). (b) Brand H., Capriotti J. A. and Arnold J., Organometallics., 13, 4469(1994). (c) Brand H.and Arnold J., Organometallics., 12, 3655(1993). (d) Arnold J. A., Johnson S. E., Knobler C. B.and Hawthorne M. F., J. Am. Chem. Soc., 114, 3996(1992).
- (a) Kim H., Whang D., Kim K.and Do Y., Inorg. Chem., 32, 360(1993).(b) Ryu S., Whang D., Kim J., Yeo W.and Kim K., J. Chem. Soc., Dalton Trans., 205(1993).
- (a) Buchler, J. W., Eikelmann G., Puppe L., Rohbock K., Schneehage H. H.and Weck D., Liebigs Ann. Chem.,745, 135(1971). (b) Buchler J. W.and Schneehage H. H., Z. Naturforsch. B., 28, 433(1973). (c) Buchler J. W., Folz M., Habets H., van Kaam J.and Rohbock K., Chem. Ber., 109, 1477(1976).
- (a) Marchon J.-C., Latour J.-M., Grand A., Beiskhovsky M., Loos M.and Goulon., J. Inorg. Chem., 29, 57(1990). (b) Lecomte C., Protas J., Marchon J.-C.and Nakajima M., 34, 2856(1978). (c) Goedken V. L., Dessay, G., Ercolani C., Fares V.and Gastaldi L., Inorg. Chem., 24, 991(1985).
- (a) Woo L. K, Hays J. A., Jacobson R. A.and Day C. L., Organometallics Organomet. Chem., 190, C61(1980). (b) Berreau L. M., Young V. G.and Woo L. K., Inorg. Chem., 34, 527(1995).
- (a) Shibata K., Aida T.and Inoue S., Tetrahedron Lett., 33, 1077(1992). (b) Shibata K., Aida T.and Inoue S., Chem. Lett., 1173(1992).
- Platt J. R.and Klevens H. B., Rev. Mod. Phy., 16, 182(1944).
- Burger H., Burczyk K.and Fuhrhop J. H., Tetrahedron., 27, 3257(1971).
- Gurinovich G. P., Sevchenko A. N.and Solover K. N., Infrared Spectra of Cholorophyll and Related Compounds and Spectroscopy of Chlorophyll and Related Compounds., Translation series, United States Atomic Energy Commission.,(1971).
- Ogoshi H., Saito Y.and Nakamoto K., J. Chem. Phys., 57, 4194(1972).
- Nafie L. N., Perzolet M.and Peticolas W. L., Chem. Phys. Lett., 20, 563(1973).
- Gurinovich G. P., Sevchenko A. N.and Solovyev K. N., Spectroscopy of Chlorophyll and Allied Compounds [Spektroskopiya khlorofilla i rodstvennykh soedineniy]., Nauka i tekhnika: Minsk.,(1968).
- Gouterman M.and Gouterman M., Excited States of Matter, Vol. 2, Texas Tech University, Lubbock: Texas, 63(1973).
- Lomova T.N., Tyulyaeva E. Y.and Bachurova S. E., Russ. J. Inorg. Chem., 55, 640(2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









