Synthesis and Characterization of Some Lanthanone Complexes of Possible Biologically Active Ligands
Abdul Vudood and P. N. Saxena
Department of Chemistry, Bareilly College, Bareilly - 243 005 (India).
The template reactions of 2,4-diaminopyridine with diacetyl in the presence of Lnn+ ions produces open chain complexes as a result of Schiff base condensations. These complexes contain free ketonic groups. The reactions of these complexes with a diamine have been studied in presence of glacial acetic acid which cause ring closer and formation of macrocyclic complexes. These complexes have been characterized on the basis of elemental analyses, conductance, magnetic moment and spectral data. Based on these studies probable structures of the complexes have been proposed.
KEYWORDS:Macrocyclic; Condensation; Schiff base and ring closer
Download this article as:| Copy the following to cite this article: Vudood A, Saxena P. N. Synthesis and Characterization of Some Lanthanone Complexes of Possible Biologically Active Ligands. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Vudood A, Saxena P. N. Synthesis and Characterization of Some Lanthanone Complexes of Possible Biologically Active Ligands. Orient J Chem 2012;28(1).Available from: http://www.orientjchem.org/?p=24070 |
Introduction
Condensation reaction between carbonyl compounds and primary amines have played an important role in the synthesis of new macrocyclic ligands (1-5). Usually such reactions are carried out in the presence of suitable metal ion which may serve to direct the condensation preferentially to cyclic products or to stabilise the macrocycles once formed. The application of macrocyclic compounds in bioinorganic chemistry, catalysis, extraction of metal ions from solution and the activation of small molecules gave impetus to this phenomenon (6-8). Keeping these facts in view the present communication describes the synthesis, characterization and biological activities of Lanthanide macrocyclic complexes.
Experimental
All the chemicals and reagents used were of AR grade or equivalent purity. The chemicals 1,8-diaminonaphthalene, benzil and orthophenylenediamine were procured from Lobochemie, Rare earth nitrates were prepared from Ln2O3 by standard method. All the solvents used were of highest purity. Ethanol was double distilled in the lab before use.
Preparation of Macrocyclic Lanthanone Complexes
The metal salt was dissolved in benzene ethanol mixture. The mixture was added to ethanolic solution of the diamine and refluxed for one hour, when the mixture became turbid, glacial acetic acid was added and a clear solution was obtained which was refluxed for 12 hours when a precipitate was obtained, it was filtered, washed with hot mixture of methanol, ethanol and benzene, the solid complexes were dried in vacuum desicator.
Physiochemical Studies
The melting points were determined by open capillary method and are uncorrected. The Schiff base Lanthanide complexes were analysed for C, H and N at CDRI Lucknow. The metals were estimated gravimetrically as oxides in the Lab. Molar conductance was determined at 10-3M dilution in DMF at 250C using Elico CM 180 conductivity meter with a dip type platinum electrode. The IR spectra of the complexes were recorded in the range of 4000-400 Cm-1 in KBr discs. The electronic spectra of the complexes were recorded using Backmann – DU spectrometer. The magnetic susceptibility measurement were carried out using Gouy’s Balance. The metal complexes were also subjected to TGA to find out the coordinated or uncoordinated water molecules.
Antibacterial Activities
These were carried out using agar diffusion method. Lab cultures of the bacteria E.coli, B. subtilis, S. Auruis and P. aeruginosa were used for inoculation. Nutrient agar medium was prepared in sterelized petridishes. The surface of agar plate was inoculated with bacterial stain. The sample solution was prepared in DMSO and was added to well in the agar medium. The plates were allowed to stain for 30 minutes and kept in incubator for 24 hours. The inhibitory zones in mm were taken as measure of antibacterial activity.
Results and Discussion
The analytical data sugested 1:1 (M:L) stoichiometry for the complexes where L is the acyclic open chain product of a Schiff base condensation of two molicules of diketones with 1 molecule of diamine. Reactions of these open chain product with diamine have been studied which gave rise to macrocyclic products.
Where:
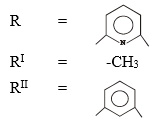
Table 1 : Elemental Analyses of Complexes
|
Molecular formula |
MolWt. |
% of C |
% of H |
% of N |
| [La (C19H19ON4).2H2O]3+3NO3 |
680 |
33.529 |
3.382 |
14.411 |
| [Ce (C19H19ON4).3H2O]3+3NO3 |
699 |
32.618 |
3.576 |
14.020 |
| [Sm (C19H19ON4).3H2O]3+3NO3 |
709 |
32.157 |
3.526 |
13.822 |
| [Tb (C19H19ON4).3H2O]3+3NO3 |
718 |
31.754 |
3.481 |
13.649 |
| [Er (C19H19ON4).3H2O]3+3NO3 |
726 |
31.404 |
3.443 |
13.498 |
The results of the elemental analyses are given in Table-1. The macro-cyclic complexes are soluble in only DMSO and DMF. Molar conductance at 250C and 10-3M dilution show these complexes to be 1:3 electrolytes.
Magnetic Moments & Electronic Spectra
The Lanthanum (III) complex is diamagnetic while Ce, Sm, Tb and Er complexes are paramagnetic in nature as expected from their respective configuration. The values are given in table-2.
TABLE-2 : Conductance and Magnetic Moment Data.
|
Molecular formula |
Molar conductance lm (p-1Cm2 mol-1) DMF |
Magnetic Moment m (B.M.) |
| [La(L)2(H2O)3] 3NO3 |
81 |
Diamagnetic |
| [Ce(L)2(H2O)3]3NO3 |
79 |
2.79 |
| [Sm(L)2(H2O)3] 3NO3 |
85 |
1.87 |
| [Tb(L)2(H2O)3]3NO3 |
89 |
9.02 |
| [Er(L)2(H2O)3] 3NO3 |
92 |
9.34 |
Electronic Spectra
La3+ has no observable visible spectra and Ce3+ has no transition in this region. The f-f transition of the complexes are characteristics of Lanthanide and are not influenced by the ligands (9). Intensity of the peaks varies according to the metal ions. The f-f bands are sharp and line like. This is because of effective shielding of the 4f orbitals by 5s, 5p octet and consequently minimum ligand field perturbation of the electronic energy level in lanthanides (10). The observed f-f transition and their assignment are given in the table below
Table 3 : Electronic Spectral Data.
|
Complexes |
Wave length (hm) |
Tentative Assignment |
| [La (L)2(H2O)3] NO3 |
– |
– |
| [Ce (L)2(H2O)3] NO3 |
– |
– |
| [Sm (L)2(H2O)3] NO3 |
445 |
6H5/2→ 4G9/2 |
|
402 |
6H5/2 → 4P3/2 |
|
| [Tb (L)2(H2O)3] NO3 |
545 |
5D4→7F5 |
|
617 |
5D4→7F5 |
|
|
675 |
5D4→7F2 |
|
| [Er (L)2(H2O)3] NO3 |
509 |
5I15/2 → 2H11/2 |
|
531 |
5I15/2→5S3/2 |
|
|
612 |
5I11/2→4F9/2 |
Infra Red Spectra
The IR spectra of the complexes show one band in the region of 1600 Cm-1 assignable to C=N stretching mode which indicate the Schiff base condensation, n (M-N) band appears in the region of 410 Cm-1 in all complexes. The common feature to all acyclic complexes is the occurrence of strong band around 1670 Cm-1 due to n (C=O). This suggested formation of open chain products containing terminal ketonic groups. This band is shifted to lower wave number by about 15-18 Cm-1 in the complexes relative to the position in free acetyl. The band at 510 Cm-1 is attributable to M-O stretching vibration. The bands around 1580, 670 and 400 Cm-1are assigned to pyridine ring deformation (11). These bands remain unchanged in the complexes, excluding the possibility of involvement of pyridine ring nitrogen in coordination.
The infra red spectra of cyclic product are quite similar to those of acyclic complexes. The only difference is that the cyclic complexes show one band near 1615 Cm-1 insted of 1670 Cm-1 which is due to C=N stretch of the newly formed azomethine linkage. The presence of water molecules in the complexes is shown by a broad absorption band in the region of 3510 – 3480 Cm-1 due to O-H stretching and sharp medium peak around 1605-1560 Cm-1 due to H-O-H bending vibrations (12).
The thermal curves of all the complexes show an exothermic peak in 182 to 2000C temperature range with mass loss which correspond to two/three coordinated water molecules.
Antibacterial Activity
The ligand was sensitive to all organisms except S. Aurius which was found to be resistant. All the Lanthanide complexes exhibited prominent activity. The higher activity of complexes may be explained on the basis of chelation theory. According to this theory the coordination of the metal reduces the polarity of the metal ion by partial sharing of positive charge with donor atoms of the ligand. The order of antibacterial activity of various complexes is La > Ce > Sm > Tb > Er.
Conclusion
Based on various studies performed the coordination number of La is 6 and forms octahedral complex, that of Ce, Sm, Tb and Er show coordination number of 7. Therefore the complexes may be represented in the following manner
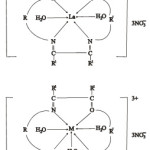 |
scheme 1 Click here to View scheme |
Acknowledgement
The authours are thankful to Dr. R.B. Singh, Head of the Department of Chemistry and Dr. R.P. Singh, Principal, BareillyCollege, Bareilly for providing facilities to carry out this research work..
References
- Melson G.A., Coordination Chemistry of macrocyclic compounds (Plenum, New York), 1979.
- Linodoy L.F., The Chemistry of macrocyclic ligand complexes (Cambridge University Press, Cambridge), 1989.
- Sargeson A.M., Coordination Chem. Rev. 151 (1996) 89.
- Colinson S.R. and Fenton D.E., Coord Chem. Rev., 148 (1996) 19.
- Tsukube H., Coord Chem Rev., 148 (1996) 1.
- Mitewa M. and Bentechev P.R., Coord Chem Rev., 135/136 (1994) 129.
- Ito I., Kato M., Yamashito M. and Ito H., J Coord Chem Rev., 15 (1986) 29.
- Mertes M.P. and Mertes K.B., Acc. Chem. Res., 23 (1990) 413.
- Seema Varghese and M.K. Muraleedharan, International Journal of applied Biology and Pharmaceutical Technology, 1( 2010) 608.
- Gajendra Kumar Pandey, Seema Srivastava, Om Prakash Pandey and Soumita Kumar Sengupta, Indian Journal of Chemistry, 37A (1998) 447-451.
- Kuppusamy Selvaraj and Chinniagounder Theivarasu, Indian Journal of Chemistry, 41A (2002) 1417-1420.
- Nikolaev A.V., Logvinen’ Ko V.A. and Myachina L.I., thermal analysis (Academic Press New York) 1969.

This work is licensed under a Creative Commons Attribution 4.0 International License.









