Study of 2-(piperidine-1-ylmethyl)cyclohexanamine Structure Synthesis Based on Oxime Protected Compounds in Mannich Mechanism
Milad Taheri1, Reza Soleymani 2, Bita Hosn1, Halimeh Rajabzadeh3 and Mohammad Raouf Darvish1
1Department of Chemistry, Shahre-Rey Branch, Islamic Azad University, Tehran, Iran.
2Young Researchers Club, Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
3Department of Chemistry, Dezful Branch, Islamic Azad University, Dezful, Iran.
2-(piperidine-1-ylmethyl)cyclohexanone (PMC), 2-(piperidine-1-ylmethyl)cyclohexanone oxime (PMCO) structures and also 2-(piperidine-1-ylmethyl) cyclohexan amine (PMCA) structure were extracted using experimental methods of Mannich reaction, based on oxime protected compounds. The given structures were purified at the end of syntheses process, by chromatography column. Related structures were identified by using 13C-NMR, 1H-NMR, FT-IR and mass spectroscopy. Attained results showed that using oxime structure as an intermediate causes to increase the yield and also to increase reaction ratio attain final product. However, all the attained spectra approved existence of intermediate and the final product. In following, thermodynamic parameters for the products including energetic levels, HOMO and LUMO parameters, chemical hardness, electrophilicity and chemical potential for this structures were calculated by using the density functional theory in method and B3LYP/6-311G(d,p) level of theory.
KEYWORDS:DFT; Electrophilicity; Mannich reaction; Oxime; Synthesis
Download this article as:| Copy the following to cite this article: Taheri M, Soleymani R, Hosn B, Rajabzadeh H, Darvish M. R. Study of 2-(piperidine-1-ylmethyl)cyclohexanamine Structure Synthesis Based on Oxime Protected Compounds in Mannich Mechanism. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Taheri M, Soleymani R, Hosn B, Rajabzadeh H, Darvish M. R. Study of 2-(piperidine-1-ylmethyl)cyclohexanamine Structure Synthesis Based on Oxime Protected Compounds in Mannich Mechanism. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23961 |
Introduction
Victor mir prepared oximes for the first time1. One of the important applications of oximes, is using these compounds at pharmacy2,3. These compounds are broadly used as carbonyl derivatives and or carbonyl group protective factors for preparing organic materials. These compounds have a high value for the organic chemists for good stability and easy preparation, that various methods were reported for their protection removal4,5. However, these compounds are as the important intermediates in organic chemistry that are applied at preparing amines or lactams. This group, holding the different biological and pharmacology characteristics2,3, has the broad application at biosynthesis, agricultural, and pharmacy processes. These groups can be used in preparing ligand in unsymmetrical synthesis and lactams synthesis4 and amines preparation5 beside of using amine and oxime functional groups. However, these compounds are applied at complexometric process and the various complexes synthesis. Some factors that lead to value oximes characteristic, is these material application at other material synthesis6-16. These compounds are used to identify aldehydes and ketenes. Because of oximes are easily inverted to functional groups and are broadly applied at other material synthesis, these materials produce base characteristics in acidic environment and concentrated acid, often oximes after formation at water phase pH=7, if being solid, deposit17. Preparation method of these materials is attained from ammonia reaction and its derivatives with aldehydes and ketenes, some solid compounds usually applied for identifying aldehydes and ketenes and attained compounds have higher sustainability than original material18.
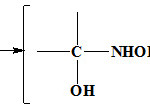 |
Figure 1:First step :oximes formation aggregation mechanism. |
Reaction continues follows the bellow figure.
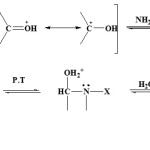 |
Figure 2: Second step :oximes formation mechanism. |
Solution must be acidic, enough to be protic the significant part of carbonyl compound. In this reactions kind, the most suitable pH that provides the highest reaction rate, depends on nucleophilic base power of N and as a result on cumulative reactor kind. But oximes have other applications such as using oximes at heterocyclic compounds synthesis and also active esters hydrolysis by oxime protected polymers that depend on macro molecule characterizes. However, oximes, have different spatial chemistry depended on the reaction kind19-21, that the structures spatial chemistry quality is effective at productions formation amount.
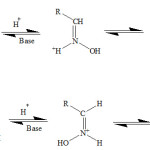 |
Figure 3: Show of syn to anti isomers convention steps in oximes |
Used oximes at amine compounds synthesis, According to high application with respect to efficacy of these compounds22,23 to examine the performance manner of these compounds at amines synthesis, Using synthesized amines has great application at manufacturing paint, fiber and medical tools24. Then, synthesized amine structure 2-(piperidine-1-ylmethyl) cyclohexnamine (PMCA) that this structure mechanism follow mannich reaction mechanism and before its synthesis, attained oxime structure as an intermediate.
 |
Figure 4: How to Mannich mechanism show. |
The products structure depends on the substrate rapture and amine part can be aliphatic or aromatic amines of kind 1 or 221,24. At continuing, examined all the synthesis structures including the raw materials, intermediate and product material in respect of thermodynamic parameters ad compared electrophilicity, chemical hardness, chemical potential and max amount of electronic charge transfer for them.
The electrophilicity index, which measures the stabilization in energy when the system acquires an additional electronic charge, ΔN, from the environment is given by Eq. 1 and is presented in terms of the electronic chemical potential, μ (the negative of electronegativity, χ) and the chemical hardness, η. Both quantities may be approximated in terms of the energies of frontier molecular orbital (εHOMO and εLUMO) as μ= (εH +εL)/2 and η=εL -εH (Eqs. 2 and 3). Electrophilicity can also be approximated in terms of the ionization potential (I) and electron affinity (A) (Eq. 1 and 2)25.
ω=μ2/2η=χ2/2η (1)
χ=-μ=-(бE/бN)V(r) ≈(I+A)/2≈-1/2 (εHOMO+εLUMO) (2)
η=(б2E/бN2)V(r) =(I-A)≈(εLUMO-εHOMO) (3)
High values of μ and low values of η, characterize a good electrophone species. The maximum amount of electronic charge, ∆Nmax, that the electrophone system may accept is given by Eq. 4as25.
∆Nmax=-μ/η (4)
Thus, while the quantity of ω describes the propensity of the system to acquire additional electronic charge from the environment, the quantity of ∆Nmax describes the charge capacity of the molecule25.
Experimental and Theoretical Details
General methods
All of the raw materials needed for doing the reaction were purchased from Germany Merk company and means and system (apparatus) needed for doing he reaction is as follow. Scale with sensitivity 0.001 gram, Japanese model ANDGF-300, mixer and heater Heidolphm R 3004 Safty model, TLC (chromatography papers on Merk thin layer) Art no: 1: 0554, melting point measuring apparatus, electro thermal, glass means and materials drier apparatus, Memmert (oven), vacuum pump C-55-JXHRL/ Emerson model and different glass means.
1H-NMR and 13C-NMR spectra were recorded with Bruker Avance 300 spectrometer with the processing software XWINNMR version 3.1. Chemical shifts are reported on 1 scale relative to TMS. The FT-IR spectra were measured by a PerkinElmer spectrum 1420 spectrometer in the frequency range of 4000–400cm−1 using KBr discs. The IR spectra were recorded at room temperature at the spectral resolution of 1 cm−1.
Synthesis
Preparation of 2-(piperidine-1-ylmethyl) cyclohexanone (PMC)
In a 250-ml two-necked flask balloon, 17.67 g (180.0 mmol) cyclohexanol, 10.94 g (90.0 mmol) piperidine hydrochloride and 2.70 g (90.0 mmol) para formaldehyde with 2cc acetic acid were put. While by magnetic mixer up 90 0C were isolated. The attained sediment was put with 20cc acetone for 10 min at reflex condition. After filtering the sediment, we washed by acetone, for this way, purified sediment was prepared. After that put the deposit in mortar at 40 0C to be dried completely. According to this way, a white deposit with melting point 230-229 0C was attained. At this stage, reaction output is 85%. Given deposit was solved in water and while coding by ice and stirring, added 30% NaOH and made it alkaline up pH 12. Then, washed the given solution 3 times and each time by 40 cc ether. For dehydration, Na2SO4 was added to the attained ether phase ad rested for one hour and frequently stirred with a glass bar. Ether phase was transferred to the operator rotary balloon by a funnel which has been set the cotton on its mouth, till its ether was evaporated at up 20 0C. Therefore 11.4 g, PMC with reaction output 65% was attained.
A yellow liquid; mp 229-230 0C (decomp) (hexane –Di ethyl ether); IR (KBr- cm-1): 2857- 2935 (Stretch CH), 1709-1717 (Stretch C=O), 1443 (Bend CH2); 1H-NMR (500 MHz, CDCl3 ) δ 1.2- 2.11 (19H, m, Aromatic-H), 2.18- 2.29 (7H, m, CH2), 2.64- 2.68 (2H, m); 13C-NMR (CDCl3) δ 24.6, 24.9, 26.3, 28.4, 33.3, 42.3, 48.9, 55.2, 58.5, 212.9.
Preparation of Oxime from 2-(piperidine-1-ylmethyl) cyclohexanone (PMC)
In a two-necked flask 250 ml flask, 0.46 g (2.3 mmol) from PMC at 15 cc ethanol 96% was resolved. Then, 0.40 g (5.7 mmol) hydroxyl amine hydrochloride and 0.80 g (5.8 mol) di hydrate sodium acetate was added. An opaque mixture was attained, added to it water drop-to-drop (about 3cc) to be transparent, set the reaction mixture for 2 hrs at the reflux condition and while coding perfectly, added to it 25cc water, and by adding 10% soda to it and controlling by pH paper, measure alkali, at this time, considerable amounts of white-color deposit are formed for completing formation of deposit, put it in the refrigerator for one day and night and after it, filtered it by Buchner funnel and after washing with distilled water and drying completely with methanol, do crystallization. At course of this reaction, 0.42 white-color oxime crystals were formed with output 84% and melting point 118 0C.
A white solid; mp 118 0C (decomp) (hexane –Di ethyl ether); IR (KBr): 3402 (OH), 2857- 2932 (Stretch CH), 1644 (C=N), 1444 (Bend CH2), 1041 (C-O), 942 (N-O); 1H-NMR (500 MHz, CDCl3) δ 1.42- 1.62 (12H, m, Aromatic-H), 2.29- 2.40 (6H, m), 2.52- 2.54 (1H, m, CH), 9.73 (1H, s, OH of Oxime); 13C-NMR (CDCl3) δ 23.78, 24.1, 24.8, 26.1, 26.53, 31.86, 39.65, 55.34, 60.26, 161.79; MS m/z 210, 211 (28.69), 209 (14.80), 193 (26.58), 98 (100), 81 (4.80), 70 (15.87), 55 (30.35), 41 (50.04).
Reduction of 2-(piperidin-1-ylmethyl)cyclohexanone oxime(PMCO)
At the first, put 2.09 g (10.0 mmol) oxime with 100cc methanol and 4,75 g (20.0 mmol) NiCl2.2H2O in a 250cc flask and for reason of being calorific of reduction reaction, ice bath was prepared. The solution was green color for existence of Nickel and chlorine then was added 1.89 g (50.0 mmol) NaBH4. Reaction solution becomes dark brown-black. Reaction advancement was control by TLC on alumina with solvent of 50% ether-petroleum ether. After 10 hrs, reaction was completed and attained deposits were filtered under vacuum. Then methanol was isolated under vacuum and 20% KOH was added to the result enough to be alkali. By increasing KOH, solution color was green. After one hour stirring, attained deposits were washed filtered under vacuum, and were washed by water and Di-ethyl-ether, Then extract the result several times by diethyl ether. Add the saturated sodium chloride to the attained watery phase and were extracting with diethyl ether. Continue to extraction several times till complete the purification, then it was dried on sodium sulfate for one hour. By sedating diethyl ether by rotary 1.4 g un-pure amine was attained. A black-brown liquid; mp 200-210 0C (decomp) (ether – oil other).
Purification of 2-(piperidin-1-ylmethyl)cyclohexanamine
For purifying amine required, two methods were used: first method, for purifying the attained amine, hydrate chloride salt was used. For this work, at the first, 10% HCl enough to be acidic was added, and was stirred for more than one hour. At this manner, amine was entered to aqueous phase and other impurities remain at organic phase. (In this system, oxime, also, because of holding OH was protic, but it was attempted to complete, as possible as, reaction). Result was extracted with ether, then added 10% soda to aqueous phase and it was stirred less than 1 hour. In this manner, amine was gone out of salt manner, and become as organic compound and then it was extracted with ether and organic phase was isolated from aqueous phase and was dried on sodium sulfate. This method doesn’t have a suitable output. Second method using chromatography with alumina stable phase. In this method, alumina powder with 50% solvent at ether ethyl-oil ether was used that oxime doesn’t act and isolated from amine product, well. Attained pure amine is 0.45 g with output 24%.
A black- brown liquid; mp 198- 200 0C (decomp) (ether – oil other); IR (KBr): 3273-3252 (NH2), 2800-2930 (Stretch CH), 1446 (Bend CH2), 1100-1200 (C-N); 1H-NMR (500 MHz, CDCl3 ) δ 1.14-1.86 (m, 11H, Aromatic H), 2.22-2.23 (dd, 4H, CH2), 2.25-2.30 (4H, s, NH2), 2.02- 2.12 (1H, m, NH2 Exial Proton), 3.28- 3.32 (1H, m, NH2 Equtrial Proton); 13C-NMR (CDCl3) δ 22.55, 22.82, 24.04, 24.05, 24.80, 24.84, 25.13, 25.47, 25.72, 25.92, 28.70, 29.85, 33.99, 34.27, 39.79, 54.92, 54.99, 66.50; MS m/z 196 (100), 167 (11.3), 150 (4.8), 135 (8.1), 124 (25.8), 112 (16.1), 95 (62.5), 91 (12.9), 84 (35.8), 77 (12.9), 67 (14.5), 57 (84.37), 42 (38.7), 30 (3.2).
Used method for purifying amines-synthesized amine at each of mentioned methods is gross and was broken down, rapidly, at exposure of whether. After it, chromatography column method for purifying it was used. In this method, chromatography column with alumina stable phase and with petroleum ether solvent were used with certain percent. In this cause oxime doesn’t react and is isolated from amine product well.
Computational methods
Density functional theory (DFT) calculations of above structures were conducted in which energies, electrophilicity, chemical hardness, chemical potential, max amount of electronic charge transfer index were obtained at the B3LYP/6-311G(d,p) level. For this purpose, computer pentum IV with intel® processor of core i7, 4 GB of Ram and XP windows® are used. At first, initial structure are designed by chemoffice 2005 software26 and optimization is done with MM2 methods27. All calculations were conducted using GAUSSIAN 98W program package27.
Discussion and Results
Total mechanism process can be observed at Fig 5.
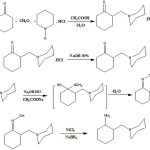 |
Figure 5: How to synthesis PMCA structure. |
At the first part of mechanism from reaction of piperidiniom hydrochloride with formaldehyde and cyclohexanone in presence of acetic acid, a pale-yellow color oily liquid named 2-(piperidine-1-ylmethyl)cyclohexanone (PMC) was attained. In this reaction named Mannich, from cyclohexanone carbonion was attained. Carbocation attained from formaldehyde with an-bond electron, piperidine reacts then by dehydration was attained which rit reacts with attained and from cyclohexanone and PMC hydrochloride salt was attained. So, output of this reaction was attained 84%. However, PMC synthesis was done by hydroxylamine and acetate sodium. Hydroxylamine is base as ammonia, and as a result, it reacts with acid and it converts into salt. Hydroxyl amine hydrochloride, is oxide harder than free bases by weather and in this way, by adding base as a base reactor commonly, sodium acetate is freed from its salt at nearby of carbonyl compound.
| Figure 6: Show of release mechanism of hydroxil amin hydrocholoride Click here to View figure |
Used accurate condition depends on reactor base power and carbonyl-compound activity. Carbonyl material accumulation with these reactors, is commonly, done by on electrophilic catalytic which is commonly a proton. Catalytic role is being coordinated with carbonyl group to makes it ready for attacking of a nucleophilic. Attacking with nitrogen of a nucleophilic and then exchanging a proton and at the end a reaction was done into deleting and leads to create a given product. In this reaction, sodium acetate undertakes to release hydroxylamine from its hydrochloride salt and also to regulate suitable pH for forming oxime. Released hydroxylamine also performs on increase attack by own free nitrogen electrons pair. Among advantages of this reaction, can refer to high output of this reaction that is 84%, and it shows melting point 117-118 0C. Its Rf at petroleum ether and diethyl ether is 0.53 (50-50). Studding approve IR, mass and NMR spectrums of attained product. Attained results for mass spectroscopy have been shown at Fig 7.
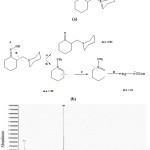 |
Figure 7: Spectrum show and how to obtained results qf mass spectrometry for PMC. |
However, in continuing, by reducing PMC at this reaction, reduction (regeneration) action has been performed by sodium borohydride, in presence of NiCl2 catalytic for converting oxime group to amine hydride ion attained is able to attach from directions equatorial and axial, till by this, it produces sin and anti for us. At presence of NiCl2, it prefers to attack to equatorials; as a result, amine group main products are at axial position (Fig 8.)
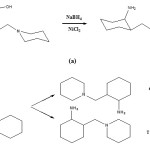 |
Figure 8: Show of how to reduction of PMC. |
The reaction, in presence of NiCl2 and NaBH4 are performed more than equivalent at methanol environment without water. At the first, reaction solution is green for presence of NiCl2, that as soon as, NaBH4 enters to it, it becomes dark brown. Reaction end with TLC are determined over stable alumina phase and 50% solvent of di ethyl ether petroleum ether and has Rf:0.54 spectroscopic results approve above cases. However, attained results for mass spectroscopy, have also been shown at below (Fig 9.).
 |
Figure 9:.how of spectrum and how to obtained results of mass spectrometry for PMCA structure |
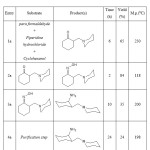 |
Table 1: Show of starting materials type and products, reaction time and obtained yield amount. |
As shown at fig 9. At m/e=96 by removing one hydrogen is converted into m/e=95 that, at continuing, we will have m/e=122. However, attained results, have been reported by momentum function theory calculations at theory of the level B3LYP/6-311G(d,p) at table 1.
Attained results for chemical roughness, chemical potential, electrophilicity and max amount of electronic charge transfer have been reported at table 2. Obtained results show that changing these parameters strongly depends on HOMO and LUMO amounts and this factor causes to access to various amounts at three compounds.
Table 2: Electronic energies (kcal.mol−1), zero-point vibrational energies (kcal.mol−1), rotational constants (GHz), heat capacities (cal.mol-1.K-1), entropies (cal.mol-1.K-1), dipole moment (Debye) , molecular orbitals energies (εHOMO and εLUMO, eV), electronic chemical potential, μ (eV), chemical hardness, h (eV), electrophilicity, ω (eV) and maximum amount of electronic charge transfer for PMC , PMCO and PMCA structures.
|
Structure |
1a |
2a |
3a |
|
Method |
B3LYP/6-311G(d,p) |
||
|
Total energy |
-376345.151 |
-411044.162 |
-364606.374 |
|
ZPVE |
199.301 |
209.812 |
222.268 |
|
Rotational constant |
1.546 |
1.058 |
1.310 |
|
|
0.335 |
0.314 |
0.353 |
|
|
0.305 |
0.272 |
0.322 |
|
Entropy |
|
|
|
|
Total |
116.064 |
121.988 |
117.338 |
|
Translational |
41.711 |
41.932 |
41.727 |
|
Rotational |
31.982 |
32.538 |
32.044 |
|
Vibrational |
42.371 |
47.518 |
43.566 |
|
|
|
|
|
|
Heat capacity |
53.131 |
58.158 |
56.282 |
|
Dipole moment(D) |
2.469 |
0.565 |
1.085 |
|
HOMO |
-0.211 |
-0.208 |
-0.212 |
|
LUMO |
-0.021 |
0.001 |
0.036 |
|
Chemical potential(μ) |
-0.116 |
-0.103 |
-0.087 |
|
Chemical hardness(η) |
0.189 |
0.209 |
0.248 |
|
Electrophilicity(ω) |
0.035 |
0.025 |
0.015 |
|
ΔNmax |
0.615 |
0.491 |
0.351 |
Conclusion
Prepared 2-(pipridine-1-ylmethyl)cyclohexanone (PMC) structure by using para formaldehyde, Piperidine hydrochloride and Cyclohexanol structure. Product of this stage, was converted by using hydroxylamine and acetate sodium into 2-(piperidine-1-ylmethyl) cyclohexanone oxime (PMCO). At next stage, 2-(piperidine-1-ylmethyl) cyclohexanamine (PMCA) was prepared from oxime reduction with sodium born hydride reactor, in presence of, NiCl2. Oxime structure is produced as an intermediate, and at all stages, each of obtained materials are is recognizable. Advantages of this method of synthesis are less expensive and higher rate at doing the reaction than other methods that it is due to the presence of oxime during this process that its converting into amine group for producing PCM causes to increase the reaction output. Thermodynamic examinations also approve this, that PMCO structure has the lowest energy level in regarding to energetic, and it will be more sustainable than the other compounds obtained.
References
- K. Flick, E. Frankus, E. Friderichs, Arzneirn.Forsch, 28: 107 (1978).
- K. VonThiele, K. Posselt, H. Offermans, K. Thiemer, Arzneim.Forsch, 30: 747 (1980).
- F. Worek, H. Thiermann, L. Szinicz, P. Eyer, Biochemical Pharmacology, 68: 2237 (2004).
- J.R. Dimmok, S.K. Raghavan, B.M. Logan, G.E. Bigan, Eur.J.Med. Chem, 18: 249 (1983).
- H. Bundgaard, Methods in Enzymology, 112: 347 (1985).
- K.V. Luzyanin, M. Galanski, V.Y. Kukushkin, D.A. Garnovskii, A.J.L. Pombeiro, Inorganic Chemical Acta, 361: 1738 (2008).
- J. Wang, J. Wu, N. Tang, Inorganic Chemistry Communication, 10: 1493 (2007).
- N.M.H. Salem, L. El-Sayed, M.F. Iskander, Polyhedron, 27: 3215 (2008).
- I.O. Fritsky, J. Świątek-Kozłowska, A. Dobosz, T.Y. Sliva, N.M. Dudarenko, Inorganic Chemica Acta, 357: 3746 (2004).
- A.R.S. Ferwanah, A.M. Awadallah, B.M. Awad, N.M. El-Halabi, R. Boese, Inorganic Chemica Acta , 358: 4511 (2005).
- J. Martinez, I. Aiello, A. Bellusci, A. Crispini, M. Ghedini, Inorganic Chemica Acta, 361: 2677 (2008).
- M.S.S. Babu, K.H. Reddy, P.G. Krishna, Polyhedron, 26: 572 (2007).
- V. Dhayal, N. Sharma, V. Sharma, R. Bohra, J.E. Drake, C.L.B. MacDonald, Polyhedron, 26: 3168 (2007).
- H. Yin, L. Quan, L. Li, Inorganic Chemistry Communication, 11: 1121 (2008).
- Sh.A. Shaker, E-Journal of Chemistry, 7: 580 (2010).
- S.K. Shingadia, K.K. Desai, E-Journal of Chemistry , 4: 97 (2007).
- J. McMurry, Organic Chemistry, 7th Ed, (2008).
- J. Wilken, S.B.H. Kent, Current Opinion in Biotechnology, 9: 412 (1998).
- W. Werner, W. Jungestand, W. Gutsche, D. Wohlrabc, Phannazie, 32: 341 (1977).
- M. Tramontini, L. Angiolini, N. Ghedini, Polymer, 29: 771 (1988).
- B.M. Trost, Fleming , Pergam&rl Pre.Newyork, 1thEd, (1991).
- M. Javanbakht, A. Shabani-Kia, M.R. Darvish, M.R. Ganjali, M. Shamsipur, Analitica Chimica Acta, 408: 75 (2000).
- Y. Abdoh, M.R. Darvish, Journal of Applied Chemistry, 17: 256 (1967).
- K.V. Patel, A. Singh, E-Journal of Chemistry, 6: 281 (2009).
- R.G. Parr, L.V. Szentpaly, S. Liu, J. Am. Chem. Soc, 121: 1922 (1999).
- Chemoffice ultra 05, cambridge soft , all rights reserved, (2006).
- Gaussian 98 software package, Gaussian Inc, Wallingford, CT, (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









