Preparation and Biological Evaluation of 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines Derivative
Parthiv K. Chaudhari
Chemistry Department, Shri R.R.Lalan College, Bhuj-Kutch-370001, Gujarat, India
The preparation of the 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines derivative (V a-m) have been undertaken by the cyclocondensation of aryl aldehyde, urea and 1-N-phenyl-3-amino-5-pyrazolone. The constitution of the product (V a-m) has been characterized by using elemental analyses, IR and PMR spectral data. The products (V a-m) were assayed for their in vitro biological assay like antibacterial activity towards Gram and Gram negative bacterial strain and antifungal activity towards Aspergillus Niger and Candida albicans at different concentration for their MIC values, the biological activities of the synthesized compounds were compared with standard drugs. Some of the obtained compounds showed the interesting antimicrobial activity comparable to standard drugs like amplicillin, chloramphenicol, amoxicillin, ciprofloxacin, norfloxacin and griseofluvin...
KEYWORDS:pyrazolo [3, 4-d] pyrimidines; antimicrobial activity and antituberculosis activity antimycobacterial activity
Download this article as:| Copy the following to cite this article: Chaudhari P. K. Preparation and Biological Evaluation of 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines Derivative. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Chaudhari P. K. Preparation and Biological Evaluation of 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines Derivative. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24010 |
Introduction
Extensive studies have been made in the field of synthesis of pyrazol [3, 4-d] pyrimidines [1-10] Consideration research has been under taken to extend activity and reduce toxicity of pyrazolo [3, 4-d] pyrimidine. The specific biological activities have been Hyperuricemia[11], Protozoacidal[12], Hypoglycemic[13], Antimalerial[14], Anticancer[15], Analgesic[16],Antipyretic[16] , Aniinflammatory[16], Antimycotic agent[17], Xanthine oxidaseinhibitor[18], Antitumor[19], Anti-HIV[20], Anxiolytic agent[21], Cardiovascular[22], Antileishmanial[23],Tranquilizer[24],Bloodsugar lower agent[25], Anticonvulant[26], Antiviral[27], Antiallergic[28].
The product(Va-m) were assayed for their in vitro biological assay like antibacterial activity towards Gram positive and Gram negative bacterial strain and antifungal activity towards Aspergillus nigor and Candida albicans at different concentration for their MIC values. The biological activities of the synthesized compounds were compared with standard drugs. [Table II]. The physical constant, antimicrobial and antimicobacterial activities of compounds (Va-m) recorded in Table II respectively.
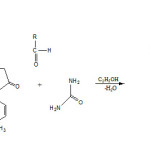 |
Scheme 1 Click here to View scheme |
Materials and Methods
Melting points were determined in open capillary tubes and are uncorrected. Formation of the compounds was routinely checked by TLC on silica gel-G plates of 0.5 mm thickness and spots were located by iodine. IR spectra were recorded Shimadzu FT-IR-8400 instrument using KBr pellet method. Mass spectra were recorded on Shimadzu GC-MS-QP-2010 model using Direct Injection Probe technique. 1H NMR was determined in DMSO-d6 solution on a Bruker Ac 400 MHz spectrometer. Elemental analysis of the all the synthesized compounds was carried out on Elemental Vario EL III Carlo Erba 1108 model and the results are in agreements with the structures assigned.
General Method for Synthesis of 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines derivative (Va-m)
A mixture of 3-amino-1-p-tolyl-1H-pyrazol-5(4H)-one (0.01 mole), urea (0.01 mole) and aldehyde (0.01mole) in ethanol (30ml) under reflux condition for three hours. The reaction mixture was kept at room temperature for 2 hrs. The product was isolated and crystallized from a suitable solvent to give the desire product. The Physical data were recorded in Table-1
Preparation of 3-amino-4, 5-dihydro-4-(4-(methylthio) phenyl)-1-p-tolyl-1H-pyrazolo [3, 4-d] pyrimidin-6-ol (V-l)
A mixture of 3-amino-1-p-tolyl-1H-pyrazol-5(4H)-one (1.88 gm, 0.01M), urea(0.60gm,0.01M) and 4-(methylthio)benzaldehyde (1.52 ml, 0.01mole) in ethanol (30ml) under reflux condition for three hours. The reaction mixture was kept at room temperature for 2 hrs. The product was collected and recrystallized from an ethanol: dioxan (2:1).
3-amino-4, 5-dihydro-4-(4-(methylthio) phenyl)-1-p-tolyl-1H-pyrazolo [3, 4-d] pyrimidin-6-ol (V-l)
IR: 3030(C-H) str. Aromatic), 1512 (C=C ring skeletal vib. Of pyrimidine) ,1456(C=N ring skeletal vib. pyrimidine), 3030(C-H str.),1178((-C-H i.p.def.), 2920(C-H asym.), 2852(C-H sym.), 1382(C-H def. sym.), 3435(N-H str.), 3373 (N-H str.), 1588 (N-H def.) ,1311 (C-N str.), 1662 (C=N str. of pyrazol), 1583 (N-N def. of pyrazol), 1178(C-N str. of Pyrazol),3325(-OH Str.)
1 H –NMR (DMSOd6+ CDCl3, δ ppm): 2.56(3H,-CH3), 2.47(3H,-SCH3), 5.95(1H, -CH), 6.71-8.70(16H, Ar-H+NH+NH2+OH)
MASS spectra: The mass spectrum fragmentation shows molecular ion (M+) peak at m/z=365.45was consistent with molecular formula C19H19ON5 OS
3-amino-4, 5-dihydro-4-(4-methoxyphenyl)-1-p-tolyl-1H-pyrazolo [3, 4-d] pyrimidin-6-ol (V-h)
IR: 3114(C-H) str. Aromatic), 1514 (C=C ring skeletal vibe. Of pyrimidine), 1459(C=N ring skeletal vib. pyrimidine), 2935(C-H str. asym.), 2845(C-H sym.), 1370(C-H sym.), 1460(C-H def. asym.), 3473(N-H str.), 3133 (N-H str.), 1587N-H def.) , 1370 (C-N str.), 1648 (C=N str. of pyrazol), 1625 (N-N def. of pyrazol), 1165(C-N str.of Pyrazol).
1 H –NMR (DMSOd6+ CDCl3, δ ppm): 2.45(3H,-CH3), 3.73(3H, 3-OCH3), 5.19 (1H, -CH), 6.80-7.02(12 H, Ar-H+NH+NH2+OH),
MASS spectra: The mass spectrum fragmentation shows molecular ion (M+) peak at m/z= 349.38was consistent with molecular formula C19H19N5O2
3-amino-4, 5-dihydro-4-(4-hydroxyphenyl)-1-p-tolyl-1H-pyrazolo [3, 4-d] pyrimidin-6-ol (V-j)
IR: 3004(C-H) str. Aromatic), 1504 (C=C ring skeletal vib. Of pyrimidine) ,1456(C=N ring skeletal vib. pyrimidine), 2925(C-H str. asym.), 2837(C-H sym.), 1365(C-H sym.), 1454(C-H def. asym.), 3471(N-H str.), 3135 (N-H str.), 1581 (N-H def.) ,1365 (C-N str.), 1651 (C=N str. of pyrazol), 1620 (N-N def. of pyrazol), 1166(C-N str.of Pyrazol).
1 H –NMR (DMSOd6+ CDCl3, δ ppm): 2.35(3H,-CH3), 5.0(1H, -OH) 5.62 (1H, -CH), 6.61-7.13(12H, Ar-H+NH+NH2+OH),
MASS spectra: The mass spectrum fragmentation shows molecular ion (M+) peak at m/z=335.5 was consistent with molecular formula C18H17N5 O2.
Conclusion
It was interesting to note that the reaction occurred immediately. This work demonstrates a very simple and efficient method for the synthesis of a well functionalized pyrazolo [3, 4-d] pyrimidines of biological importance in excellent yields.
Table 1:Physical data 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines derivative (Va-m)
|
Sr. No. |
R |
Molecular Formula |
M.W. |
m.p. OC |
Yield (%) |
Rf Value |
% of Nitrogen |
|
|
Calcd. |
Found |
|||||||
|
V a |
C6H5 |
C18H17N5O |
319.36 |
240 |
68 |
0.68 |
21.80 |
21.75 |
|
V b |
2-Cl-C6H4 |
C18H16ClN5O |
353.80 |
252 |
72 |
0.63 |
19.69 |
19.63 |
|
V c |
4-Cl-C6H4 |
C18H16ClN5O |
353.80 |
260 |
67 |
0.59 |
19.69 |
19.65 |
|
V d |
3-NO2–C6H4 |
C18H16N6O3 |
364.35 |
271 |
72 |
0.53 |
22.95 |
22.90 |
V e |
2-NO2-C6H4 |
C18H16N6O3 |
364.35 |
245 |
78 |
0.58 |
22.95 |
22.90 |
|
V f |
3-C6 H5-O-C6H4 |
C24H21N5O2 |
411.45 |
249 |
64 |
0.70 |
16.94 |
16.90 |
|
V g |
2-OCH3-C6H4 |
C19H19N5O2 |
349.38 |
259 |
58 |
0.55 |
19.94 |
19.91 |
|
V h |
4-OCH3-C6H4 |
C19H19N5O2 |
349.38 |
250 |
65 |
0.53 |
19.94 |
20.89 |
|
V i |
2-OH-C6H5 |
C18H17N5 O2 |
335.35 |
240 |
72 |
0.51 |
18.56 |
20.51 |
|
V j |
4-OH-C6H5 |
C18H17N5 O2 |
335.35 |
255 |
75 |
0.49 |
18.56 |
20.50 |
|
V k |
C6H4-CH=CH |
C26H23N5O |
421.49 |
265 |
62 |
0.62 |
20.17 |
19.11 |
|
V l |
4-CH3S-C6H4 |
C19H19ON5 OS |
365.45 |
219 |
71 |
0.63 |
19.07 |
22.02 |
|
V m |
α- C4H3O |
C24H21N5 O2 |
385.45 |
290 |
77 |
0.61 |
22.50 |
16.45 |
TLC Solvent systems: Acetone: Benzene= 1:9
Antimicrobial Activity
Antimicrobial was carried out by using cup-plate method .which has been described as under. Antibacterial Activity
Gram positive bacteria were grown in nutrient broth and Gram negative bacteria in Peptone water (PW, 1% bacteriological peptone and 0.5% NaCl) for 24 hours; this gave an optimum growth of the test bacteria. Each purified compound was dissolved in DMF sterilized by filtration by using sintered glass filter and stored at 40C.Each agent was then added to molten nutrient agar in the following concentration(µg/ml): 0 (control), 25,50,100,200,500,800and poured into sterile Petri dished. The pH of the media was maintained at 7.2-7.4. The inoculums consisted of an overnight growth broth culture of a bacterium diluted in such a manner that a 2mm (internal diameter) loopful of the culture contain 100 colony-forming units (CFU). These were then spot inoculated on nutrient agar plates containing increasing amount of a compound, incubated at 370C up to 24 hrs. for determination of the minimum inhibitory concentration (MIC) .The antibacterial activity of the compounds (Va-m) was compared with known standard reference drugs like Ampicillin, Ciprofloxacin, Chloramphenical, Griseofulvin, at same concentration. The moderate and comparable antibacterial activities of compound are recorded.
Antifungal Activity
Aspergillus Niger MTCC-282 and Candida albicans MTCC-227 were employed for testing fungicidal activity using cup plate method. The cultures were maintained on Sabouraud’s agar for72 hours this gave an optimum growth of the test fungal spores Each purified compound was dissolved in DMF sterilized by filtration by using sintered glass filter and stored at 40C.Each agent was then added to Sabouraud’s agar in the following concentration(µg/ml): 0 (control), 25,50,100,200,500,800 and poured into sterile Petri dished.. The inoculums consisted of an overnight growth broth culture of a bacterium diluted in such a manner that a 2mm (internal diameter) loopful of the culture contain 105 colony-forming units (CFU). These were then spot inoculated on Sabouraud’s agar plates containing increasing amount of a compound, incubated at 370C up to 48 hrs. For determination of the minimum inhibitory concentration (MIC) .The MIC value of test solutions are recorded in Table No-2 and Table No-3
Table 2:Antimicrobial Activity of 3-amino-4-aryl-4, 5- dihydro-1-N-tolyl pyrazolo [3, 4-d] pyrimidines derivative (Va-m)
| Compound | R | Antibacterial activity | Antifungal activity | ||||
| S.pyogensMTCC-442 | S.aureusMTCC-96 | E.ColiMTCC-443 | B.subtillisMTCC-441 | C.alibicansMTCC-227 | A.nigerMTCC-282 | ||
|
V a |
C6H5 |
100 |
200 |
500 |
500 |
200 |
200 |
|
V b |
2-Cl-C6H4 |
100 |
50 |
25 |
100 |
25 |
50 |
|
V c |
4-Cl-C6H4 |
200 |
– |
– |
50 |
– |
100 |
|
V d |
3-NO2–C6H4 |
25 |
– |
– |
50 |
– |
– |
V e |
2-NO2-C6H4 |
500 |
500 |
– |
800 |
500 |
– |
|
V f |
3-C6 H5-O-C6H4 |
50 |
– |
500 |
500 |
25 |
800 |
|
V g |
2-OCH3-C6H4 |
– |
50 |
500 |
– |
– |
500 |
|
V h |
4-OCH3-C6H4 |
50 |
100 |
200 |
500 |
500 |
– |
|
V i |
2-OH-C6H5 |
100 |
200 |
– |
500 |
– |
800 |
|
V j |
4-OH-C6H5 |
500 |
500 |
800 |
500 |
– |
– |
|
V k |
C6H4-CH=CH |
– |
– |
500 |
500 |
– |
500 |
|
V l |
4-CH3S-C6H4 |
– |
500 |
500 |
500 |
500 |
– |
|
V m |
α- C4H3O |
– |
800 |
500 |
200 |
200 |
200 |
Table 3: Comparable Activity of compounds (V a-m) with known chosen standard drugs
|
Comparative activity of (III a-n) with known chosen standard drugs |
||||||
|
Standard Drug |
|
Antibacterial activity |
|
Antifungal activity |
||
|
S.pyogens MTCC-442 |
S.aureus MTCC-96 |
E.Coli MTCC-443 |
B.subtillis MTCC-441 |
C.alibicans MTCC-227 |
A.niger MTCC-282 |
|
|
Vd (25) |
Vb(50) |
Vb(50) |
Vc (50) |
Vb (25) |
Vb (50) |
|
|
Vf (50) |
Vg(50) |
– |
– |
|||
|
Ampicillin |
30 |
20 |
30 |
30 |
– |
– |
|
Amoxycillin |
20 |
20 |
20 |
20 |
– |
– |
|
Cifalexin |
20 |
30 |
30 |
20 |
– |
– |
|
Erythromycin |
30 |
30 |
20 |
20 |
– |
– |
|
Chlotrimazole |
– |
– |
– |
– |
20 |
20 |
|
Griseofulvin |
– |
– |
– |
– |
30 |
20 |
N.B. :(-): No activity
Acknowledgements
The author’s wish to thanks to Principal, Shree R.R. Lalan College, Bhuj for providing research facilities.
References
- Homer A. Buresh, J.med.chem.11, 81-83(1968).
- Clarke, Anthony Graham R. (Delmer Chemical Ltd.) Brit 1,284,084(Cl. Cod, A61K) 02 Aug.1972.Appl 19, 684/69, 17 Mar 1969, 9pp.Chem.Abstr, 77:152213f (1972).
- Samira A. Selam, Osama I. Abdel Salam anel magdi E.A. Zaki, Ind. J. Het .Chem., 22, 1435 (1985).
- C.J. Shishoo, T. Ravikumar ,K.S. Jain, I.S. Rathod, P. Gandhi, M.C. Satia, Ind. J. Chem., 38B,1075-85(1999).
- Peter Scheriner, Sara arwin, Manes Elicin, James Tu (USA): J.Het.Chem, 22, 1435 (1985).
- Sadao Nishigak, Misuzu Ichiba, Kiyoko Fukami; J. het .Chem.15, 359 (1978).
- Keitaro Senga, Yukako Kanamori, Hashine Kanazawa; J. Het hem. 19,759 (1982).
- Das, Prabhat K., Behera G.B.; Sahay, A.K.; Ind.J.Chem. Sec.-B, 1985 24B (4), 437-9(Eng). Chem.Abstr. 104: 148823g (1985).
- L.Lee Nord, Ganapathi.R. Ravankar, Ronald K. Robins (USA), J.Het.Chem.27, 439(1990).
- Hitachings, George H.; Talco, Elvira A… Welcome and Co) U.S. 3,519,716 (Cl. 424-251; A61 K), 07 Jul1970, Brit, Appl. 23 May 1962-23 Aug 1962, 2pp.Chem. Abstr. 104: 148223g (1985).
- Podesva. Citral; Musil, Vaclav; Scott, William; (Delmar Chemical Ltd.) Ger Offen. 2,018, 345 (Cl. Co7d), 19 Nov.1970, Brit. Appl.17 Apr. 1969; 35 pp. Chem. Abstr.74; 22888q (1971)
- Kranf, Eckart; Bock, Marianne ;( Farbenfabriken Bayer, A-G). Ger. Offen. 2, 058,500(Cl.CO7d) 31 May 1972, Chem.Abstr. 77:88523w(1972)
- Bruener, Hermann; Schulze, Ernst: Treuner, Uwe, (Chemisclie Fabrik Vonugden, A-G) Ger. Offen, 2 136,950 (Cl. Co7d) .Chem.Abstr. 76: 140860f(1972).
- Howarth, Grahum; Gainer, James; (Ciba Geigry A-G). Ger. Often. 2, 218,717 (Cl. CO7D) 16 Nov. 1972, Brit.Appl. 11, 492, 171, 27 Apr 1971; 48 pp.Chem. Abstr. 78: 43514y(1973).
- Griengl, H.; Guenfl. F. (Austrica), J.Het.Chem. 21(2).505-(1984).
- Inoue, Makoto; Okamura, Takashi; Shoji Yasuo; Hashimoto Kinji (Ostuka Pharm. Factory Inc. Japan), PCT Int. Appl. WO, 96, 32,394(Cl.CO7D487/04). 17 Oct.1996, JP Appl. 95/236, 427, 14 Sep. 1995; 61 pp. Chem.Abstr. 126, 18885 x (1997).
- Kreutzberger, Alfred; Brugcuitz, Klemens (Berlin) Arch. Pharm. (Weinheim, Ger.) 1983, 313 (11), Chem.Abstr. 94, 139746g (1970).
- Senga, Keitraro, Robins, Ronald K., J.Het.Chem. 19(6), 1567-7, 1982; Chem. Abstr., 98, 160667z (1983).
- Schwartz, Pauline M.; Dunigan, Janis M.; Maroh, John C.; Handschumacher, Robert E.(USA) , Cancer Res. 1980 ,40(6), 1885-9(Eng.); Chem. Abstr., 93 88657y (1980).
- Lak S. Jeong, J. Warren Beach, Chung K. Chu; J. Het. Chem., 30 1445(1993).
- Dusza, John P.; Albright, Jay D. U.S. 4,281,000(Cl.424-251; A61K31/505), 28 Jul 1981, App. 55, 941, 09 Jul1974; 12pp. Chem. Abstr. 95, 187293z (1981).
- Regnier, Gilbert; Canevari, Roger: Poignunt, Jean Claude (Science Union et CieSociete-Francaise de Recherche Medicale), Ger. Often. 2, 651, 789, Chem. Abstr. 87, 168097(1977).
- Y. S. Prabhakar, V.J. Ram;Ind. J. Pharm. Sci., 59(6), 296-91(1997).
- Shibutani, Naotaka; Koji, Yasuo: (Otsuka Pharmaceutical Co. Ltd. Japan), Jpn. Kokai Tokkyo Koho JP 10 273,074 [98 237, 074] (Cl. CO7D 487/04); Chem. Abstr. 129, 1260469p (1998).
- Breuer, Hermann; Schulze, Ernst, (Chemische Fabrik Von Heyden A-G), Ger. Offten. 2 140, 986 (Cl.CO7d), 09 Mar. 1972, US Appl. 69, 172, 02 Sep. 1970; 15 pp. Chem. Abstr. , 77, 34565t (1972).
- Wellcome Foundation Ltd., Jpn. Kokai Tokkyo Koho JP 79 22,393 (Cl. COD 487/04), 20 Feb. 1979, Brit. Appl. 7/30, 380, 20 Jul. 1977; 21 pp. Chem. Abstr., 90, 204158a (1979).
- Ege, Guenter; Pross, Michael; Ger.Often. DE 4, 333, 705 (Cl. CO7D 487/04) 06 Apr. 1995, Appl.02 Oct.1993; 11pp, Chem. Abstr. 123, 33098 z (1975).
- Richard J. Goebel, Alexander D. Adams, Pactricia A. J.Med.Chem., 25, 1334(1982).

This work is licensed under a Creative Commons Attribution 4.0 International License.









