Perconcentration of Ni(II) from Sample Water by Modified Nano Fiber
Ali Moghimi1* and Mohamad Javad Poursharifi2
1Department of Chemistry, Varamin (Pishva)Branch Islamic Azad University, Varamin (Iran).
2Department of Chemistry, Saveh Branch Islamic Azad University, Saveh (Iran).
A new simple and reliable method for rapid and selective extraction and determination of trace levels of Ni 2+ ion is developed. Ni ions are adsorbed quantitatively during passage of aqueous samples through nano polyacrylonitrile fiber modified. Almost all matrix elements were found to pass through the nano polyacrylonitrile fiber to drain. The retained Ni 2+ ions are then stripped from the nano polyacrylonitrile fiber with a minimal amount of thiosulfate solution as eluent and subsequently measured by atomic absorption spectrometry. Modified nano polyacrylonitrile fiber (PANF) was prepared by adding of acrylic fibers to methanolamine (MMA) with different concentration solutions. The stability of a chemically modified nano polyacrylonitrile fiber especially in concentrated hydrochloric acid which was then used as a recycling and pre-concentration reagent for further uses of Modified nano polyacrylonitrile fiber. The application of this Modified nano polyacrylonitrile fiber for sorption of a series of metal ions was performed by using different controlling factors such as the pH of metal ion solution and the equilibration shaking time by the static technique. Ni(II) was found to exhibit the highest affinity towards extraction by these Modified nano polyacrylonitrile fiber phases. The pronounced selectivity was also confirmed from the determined distribution coefficient (Kd) of all the metal ions, showing the highest value reported for Ni(II) to occur by Modified nano polyacrylonitrile fiber. The potential applications of Modified nano polyacrylonitrile fiber for selective extraction of Ni(II) to occur from aqueous solution were successfully accomplished as well as pre- concentration of low concentration of Ni(II) (60 pg ml-1) from natural tap water with a pre-concentration factor of 100 for Ni(II) off-line analysis by flame atomic absorption analysis.
KEYWORDS:Preconcentration; Ni(II); Modified nano polyacrylonitrile fiber
Download this article as:| Copy the following to cite this article: Moghimi A, Poursharifi M. J. Perconcentration of Ni(II) from Sample Water by Modified Nano Fiber. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Moghimi A, Poursharifi M. J. Perconcentration of Ni(II) from Sample Water by Modified Nano Fiber. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23947 |
Introduction
Ni at trace concentrations acts as both a micronutrient and a toxicant in marine and fresh water systems 1-8.This element is needed by plants at only very low levels and is toxic at higher levels. At these levels, Ni can bind to the cell membrane and hinder the transport process through the cell wall. Ni at nearly 40ng mL-1 is required for normal metabolism of many living organisms 9, 10. On the other hand, Ni is an important element in many industries. Thus, the development of new methods for selective separation, concentration and determination of it in sub-micro levels in different industrial, medicinal and environmental samples is of continuing interest. The determination of Ni is usually carried out by flame and graphite furnace atomic absorption spectrometry (AAS) 11, 12 as well as spectrometric methods 13, 14 .
Solid phase extraction (SPE) methods are the best alternatives for traditional classic methods due to selective removal of trace amounts of metal ions from their matrices. SPE determinations can be carried out on different efficient ways. One of the most appropriative performation features of SPE is achieved by using octadecyl silica membrane disks. SPE reduce the use of toxic solvent, disposal costs, and extraction time15-16.The octadecyl silica membrane disks involves shorter sample processing time and decreased plugging due to the large cross-sectional area of the disk and small pressure drop which allows higher flow-rates; reduced channeling resulting from the use of sorbent with smaller particle size and a greater mechanical stability of the sorbent bed17.
In our previous attempts, we modified SPE membrane disks with suitable compounds for selective determination of chromium 18-19,35 and lead20.Meanwhile, other investigators have successfully utilized these sorbents for quantitative extraction and monitoring trace amounts of lead21-23, copper24-26, silver27-28, mercury29-30 , cadmium31 , palladium32, Ce33 and UO234.
This paper describes the applications of Modified nano polyacrylonitrile fiber for selective extraction and solid phase pre-concentration of Cr (III) from aqueous and natural water samples.
Experimental
Reagents and materials
Analytical grade nitrate salts of Hg, Mn, Fe and Ni(II) litium, sodium, potassium, magnesium, calcium, strontium, barium, zinc, cadmium, lead, nickel, cobalt(II) and copper(II)of reagent grade were of the highest purity. Ultra pure organic solvents were obtained from E.Merck, Darmstat, Germany, and High Purity deionized water was used throughout the experiments and 3-chloro propyl trimethoxysilane was received from Aldrich Chemical, USA. Organic solvents were dried according to conventional methods. For all solutions double distilled water was used and the buffer solutions were prepared from 1.0M sodium acetate to which different volumes of 1.0M HCl; HNO3 were mixed and the pH-value of the resulting solution was adjusted with the use of a pH- meter.
Preparation of Modified nano polyacrylonitrile fiber
Modified nano polyacrylonitrile fiber was prepared by adding 3 g of acrylic fibers to 300 ml of methanolamine (MMA) with different oncentration solutions. The reaction mixtures were refluxed at 91ºC under stirring for 2h. The reaction product was cooled to room temperature, then the product was washed with acetone and distilled water and then air-dried. The content of the MMA groups in the fiber was calculated as follows:
EA= (W1–W0)M0/(M1W0) (1)
where EA is the content of MMA groups in the fiber(mol/g),W1 is the weight of the dry fiber after reaction (g), W0 is the weight of the dry fiber before reaction (g),M0 is the molecular weight chain unit CH2CHCN(53), AND M1 is the molecular weight of NH2(CH2)2OH[31,32].
Activation of surface Modified nano polyacrylonitrile fiber (PANF) was filtered, washed with toluene, methanol and diethyl ether and dried in an oven at 70°C for 6 h. An amount of 20.0 g of dry Modified nano polyacrylonitrile fiber (PANF) .The resulting phase was filtered, washed with toluene, methanol and finally with water several times . The phase was then dried in an oven at 60°C for 7 h.
Apparatus
The pH measurements were conducted by an ATC pH meter (EDT instruments, GP 353)calibrated against two standard buffer solutions of pH 4.0 and 9.2. Infrared spectra of Modified nano polyacrylonitrile fiber (PANF) were carried out from KBr by a Perkin-Elmer 1430 ratio recording spectrophotometer. Atomic absorption analysis of all the metal ions except Ni(II) were performed with a Perkin-Elmer 2380 flame atomic absorption spectrometer. Ni(II) determinations were performed by a Varian Spect AA-10 plus atomic absorption spectrophotometer equipped with VGA-76 vapour generation
Electro Spinning
The formation of a thin fiber via electrospinning is based on the uniaxial stretching (or elongation) of a viscoelastic jet derived from a polymer solution or melt [3]. PAN is solved in common organic solvent. The solubility of raw acrylic fibers (RAF) in dimethylformamide (DMF) was (17:83 w/w) but it was observed that the solubility of PANF-MMA was (19:81 w/w) and this is because of modification. The whole solutions were prepared by being dissolved in DMF (14:86 w/w) under stirring for several hours at room temperature. The aluminum plate were used as collector and prepared at 20630 cm2. The polymer suspension was delivered to capillary nozzle via a feed line from a syringe pump. The spinneret protruded through the center of the plate. A power supply provided upto 20 KV to the plate and the distance between the capillary nozzle and the plate was adjusted at 20 cm to obtain a stable and continuous jet.
2.4 Adsorption and Removed Processes of Metal Ions The adsorption ions onto PANF-MMA for Ni (II), Cu (II), and Pb(II) ions were investigated using the batch method. Experiments were carried out in an Erlenmeyer flask at the desired pH and 258C temperature. The flasks were agitated on a shaker for 2h. The amount of adsorbed metal was determined by the difference between the initial metal ion concentration and the final one after equilibrium [3].The concentration of ions was determined with a flame atomic absorption (FAA) spectrometer (Philips
model PU9100).
The efficiency of metal ions recovery was estimated by the sorption yield (R%) and the q (mg/g) was calculated as:
R= (C0– Ct)/C0×100 (2) q = (C0 – Ct)/G×V (3)
where C0 is the initial metal ion concentration (mg/l), Ct is the ion concentration after the adsorption period, V is the volume of solution L and G is the dry mass of the PANF-MMA fiber sample (in gram). The metal ions adsorbed on the PANF-MMAwere then removed by placing 0.1 g of metal loaded fiber in 10 ml of 1M HNO3 solution for 30 minutes [28].
Stability studies
The stability of Modified nano polyacrylonitrile fiber phases in different buffer solutions (pH 1–6) and concentrated hydrochloric and nitric acids was studied by batch equilibration. In this procedure, 500 mg of the phase was mixed with 50 ml of the selected solution in 100 ml measuring flask and automatically shaken for 5 h. The mixture was filtered, washed with 500 ml water and dried in an oven at 80°C. Around 100 mg of the treated phase was added to 1.0 ml of 0.1M Ni(II) and 9.0 ml of 0.1M sodium acetate and the mixture was shaken for 30 min by an automatic shaker. The percentage of hydrolysis of polyacrylonitrile from the surface of modified nano polyacrylonitrile fiber phases in different acidic solutions was calculated from the determined µmol g−1 value of each treated phase.
Sorption studies
Determination of metal capacity values (µmol g−1)
The determination of metal capacity of 13 metal ions, viz. Ba(II) Ca(II), Co(II), Ni(II), Cu(II), Fe(III), Hg(II), Mg(II), Mn(II), Pb(II) and Zn(II) as a function of pH was studied by the static technique. Then 100 mg of the dry phase was added to a mixture of 1.0 ml of 0.1M metal ion and 9.0 ml of the buffer solution (pH 1–6 and 0.1M sodium acetate) in 50 ml measuring flask. The mixture was then automatically shaken for 30 min, filtered, washed with 50 ml water and the unbound metal ion was subjected to complexometric titration using the proper buffer and indicator solutions and/or atomic absorption analysis. The effect of shaking time on the percentage extraction of metal ions was also studied for only Ni(II) by the static technique. In this, 100 mg of the Modified nano polyacrylonitrile fiber phase was added to 1.0 ml of 0.1M Cd(II) and 9.0 ml of 0.1M sodium acetate in 50 ml measuring flask and automatically shaken for the selected period of time (1, 5, 10, 20,25,30 and 35 min). The mixture was filtered, washed with 50 ml water and the free metal ion was determined as described above.
Determination of the distribution coefficient
About 100 mg of the Modified nano polyacrylonitrile fiber phase was mixed with 50 ml of the metal ion (1 mgml−1) in a 100 ml measuring flask and shaken for 3 h by an automatic shaker. The mixture was filtered, washed with water and diluted with 2% nitric acid solution in order to fit in the linear dynamic range of each metal ion. A standard solution for each metal ion was also prepared in a similar way.
Percentage removal of Ni(II) from aqueous solutions
One liter of Ni(II) solution, containing 10, 50 and 100 ng ml−1 was passed over a column [27] packed with 500 and 1000 mg each of Modified nano polyacrylonitrile fiber. The flow rate was adjusted to 2.0 ml min−1. The eluents were collected and 5ml was diluted with 20 ml of 2% nitric acid solution and subjected to flame atomic absorption spectrometric analysis (FAAS).
Pre-concentration of Ni(II) from aqueous and natural tap water
Two liters sample solution spiked with 20 pg ml−1 of Cr (III)in both double destilled water DDW and natural tap water were prepared and passed over a column packed with 1000 mg of Modified nano polyacrylonitrile fiber with a flow rate of 2ml min−1. Then 10 ml concentrated hydrochloric acid (10.0 M) was then passed over the phase and adsorbed metal ion to desorb the bound-Ni(II). The desorbed metal ion was directly determined by FAAS. A standard solution and blank aqueous and tap water samples were also prepared and determined for evaluation.
Results and discussion
Stability studies
The stability of the newly synthesized Modified nano polyacrylonitrile fiber phases was performed in different buffer solutions (pH 1, 2, 3, 4, 5, 6 and 0.1M sodium acetate) in order to assess the possible leaching or hydrolysis processes. Because the metal capacity values determined in Section 3.2 revealed that the highest one corresponds to Ni(II), this ion was used to evaluate the stability measurements for the Modified nano polyacrylonitrile fiber phase [14]. The results of this study proved that the Modified nano polyacrylonitrile fiber is more resistant than the chemically adsorbed analog especially in 1.0, 5.0 and 10.0M hydrochloric acid with hydrolysis percentage of 2.25, 6.10 and 10.50 for phase, respectively.
However, the use of nitric acid with different concentration values (1.0, 5.0, 10.0 M) was found to change the color of Modified nano polyacrylonitrile fiber from dark brown into reddish brown which is interpreted on the basis of chemical changes of the organic nano polyacrylonitrile modifier via oxidation. In addition, stability of phases was also confirmed from the interaction with 10.0M hydrochloric acid for more than 1 week.
This test proved a reasonable stability of Modified nano polyacrylonitrile fiber phase compared to non-treated silica gel phases judging from the color change of the two phases as well as the metal capacity values determination of Ni(II) and comparison of these with those of the original non-treated Modified nano polyacrylonitrile fiber phases.
Thus, these stability studies indicated the suitability of phase for application in various acid solutions especially concentrated hydrochloric acid and extension of the experimental range to very strong acidic media which is not suitable for other normal and selective chelating ion exchangers based on a nano polymeric matrix [9]. Finally, the Modified nano polyacrylonitrile fiber phases were also found to be stable over a range of 1 year during the course of this work.
Metal capacity in various controlling factors
The metal capacity values determined in µmol g−1 for the Modified nano polyacrylonitrile fiber in different buffer solutions were studied to evaluate the pH effect of metal ion on the extractability of the Modified nano polyacrylonitrile fiber phase. Table 1 compiles the µmol g−1 values for the 13 tested metal ions, viz) Ni(II), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Cr(III) and Pb(II). Several trends can be observed and outlined from the data given. First, is the strong dependence of µmol g−1 extracted values from the metal ion solution for most tested metal ions on the pH-value [20,25].
The maximum value was found to be mainly at higher pH-values (pH 5–6 and 0.10M NaOAc). Second, is the strong affinity of the Modified nano polyacrylonitrile fiber phase for extraction and removal of Ni(II) from aqueous solution compared to other tested metal ions, as shown by the higher µmol g−1 values by Modified nano polyacrylonitrile fiber phases(25).
Table 1: Metal capacity values determined in µmol g−1a
|
Ba |
Mg |
Ca |
Zn(II) |
Cu(II) |
Co(II) |
Fe(III) |
Mn(II) |
Hg(II) |
Pb(II) |
Ni(II) |
pH |
|
|
30 |
20 |
23 |
19 |
63 |
14 |
– |
20 |
52 |
59 |
240 |
NaOAc |
|
|
38 |
28 |
21 |
39 |
91 |
35 |
– |
58 |
36 |
45 |
210 |
6 |
|
|
29 |
29 |
35 |
20 |
75 |
50 |
10 |
69 |
23 |
33 |
137 |
5 |
|
|
20 |
15 |
30 |
10 |
60 |
25 |
44 |
50 |
15 |
25 |
58 |
4 |
|
|
13 |
10 |
25 |
5 |
45 |
14 |
30 |
28 |
10 |
12 |
39 |
3 |
|
|
6 |
5 |
15 |
00 |
25 |
10 |
26 |
18 |
8 |
7 |
22 |
2 |
|
|
2 |
00 |
2 |
00 |
14 |
5 |
6 |
13 |
3 |
3 |
10 |
1 |
|
a Values are based on n=3 with standard deviation of 4.
This behavior of Modified nano polyacrylonitrile fiber –loaded sulfur containing compounds for selective extraction and removal of Ni(II) from aqueous and natural water sample is well documented [19,20] and reported based on different governing rules [17]. Third are the notably high µmol g−1 values determined for chemically modified Modified nano polyacrylonitrile fiber phase in comparison with values found as given in Table 1. The comparison between the metal sorption properties of chemically and physically- Modified nano polyacrylonitrile fiber phases has been extensively studied [21] and the results presented in this work are consistent with the surface activity of the donor atoms responsible for metal ion interaction, sorption, extraction and selective removal. In the case of the physically adsorbed phase, some of these donor atoms are involved in physical adsorption processes with the active surface, leading to the minimization of the reactivity of such donor atoms for metal interaction and binding processes. The product, Modified nano polyacrylonitrile fiber, in this case is tuned with the active donor atoms (N) directed with the capability and accessibility for fast and direct interactions with the free metal ion present in solution. Fourth, are the general orders of metal capacity values for all tested metal ions by the two phases which are in many respects consistent and similar. Therefore, the conclusion drawn from this section can be outlined as the high superiority of phase for selective extraction of Ni(II) as well as the higher metal uptake behavior of Modified nano polyacrylonitrile fiber phase .
The effect of shaking time on the percentage extraction of metal ions at various equilibration time intervals (1, 5, 10, 15, 20, 25 min) was also studied and evaluated as µmol g−1 and correlated to that determined at 30 min shaking time. Fig. 1 represents the percentage extraction versus shaking time in min and clearly reflects the rapid exchange equilibrium between Modified nano polyacrylonitrile fiber phase and Ni(II). One minute shaking time was found to be sufficient to establish 84% of the determined µmol g−1 value at 30 min whereas 10 min shaking time led to 88% extraction. The data and results presented in this section reveal the superiority of ? Modified nano polyacrylonitrile fiber phase as previously declared in the stability studies (Section 3.1).
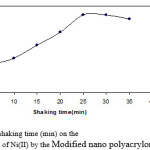 |
Figure 1: Effect of shaking time (min) on the percentage extraction of Ni(II) by the Modified nano polyacrylonitrile fiber phases.
|
Table 2: Distribution coefficient (Kd) values of various metal ions
| Metal ions | Kd | |
| Mn(II) | 89 | |
| Fe(III) | 77 | |
| Co(II) | 76 | |
| Cu(II) | 700 | |
| Zn(II) | 499 | |
| Cu(II) | 118 | |
| Ni(II) | 12600 | |
| Pb(II) | 130 | |
The distribution coefficient (Kd) data of the tested metal ions with the two newly Modified polyacrylonitrile fiber phase are summarized in Table 2. It is evident that Ni(II) is the strongest sorbed metal ion by Modified nano polyacrylonitrile fiber phase. The distribution coefficient values of Ni(II) by the loaded Modified nano polyacrylonitrile fiber phase were found to be much higher than those reported for ion exchange resins containing Modified nano polyacrylonitrile fiber derivatives [9]. In addition, the Kd values for Ni(II) by Modified nano polyacrylonitrile fiber phase were found to come on the second place after Ni(II) which behavior can be interpreted on the basis of the affinity of both nitrogen and hidroxil donor groups present in Modified nano polyacrylonitrile fiber for binding with Ni(II) [19,20]. On the other hand, the various tested metal ions as shown in Table 2 were found to exhibit lower tendency to bind with Modified nano polyacrylonitrile fiber phase judging from the comparable low distribution coefficient values determined for these metal ions. The higher Kd value for Ni(II) and the lower ones for the other metal ions, except Ni(II), provide an additional evidence for the suitability of these two newly Modified nano polyacrylonitrile fiber phase for selective extraction of Ni(II) from aqueous solutions. It is also noteworthy that the conclusion drawn from the evaluation of the Kd values by Modified nano polyacrylonitrile fiber phase is consistent with the reported data .
Percentage removal of Ni(II) from aqueous solution
The use of a column technique is a common procedure for extraction, separation and selective extraction of metal ions from various aquatic systems [10]. The column technique is characterized by major advantages over the batch or static equilibration method that is the possible application to large sample volumes [14–16]. This property enables the pre-concentration of metal ions at very low trace levels. The percentage removal of metal ions from aqueous solutions is essential for the evaluation of the method described and suggested here. This is mainly dependent on several well known factors such as the type and amount of packing stationary and mobile phases and the flow rate of the mobile phase [21]. In this study, we attempted to evaluate the percentage recovery of Ni(II) with different spiked concentrations, namely 10, 50 and 100 ng ml−1 from 1 l of 0.1M NaOAc solution by the application of two different amounts (500 and 1000 mg) of Modified nano polyacrylonitrile fiber phase packing. The results of the percentage removal of Ni(II) from aqueous solutions are presented in Table 3 which clearly demonstrate the suitability and validity of Modified nano polyacrylonitrile fiber phase for removal and extraction of Ni(II). In addition, the effect of packing amount of silica gel phase is also evident in Table 3, where the near completion of Ni(II) removal was accomplished by the use of 1000 mg phase.
Table 3 :Percentage removal of Ni(II) from aqueous solutions by Modified nano polyacrylonitrile fiber phase a
| Ni(II) spiked
(ng ml−1) |
Phase (mg) |
Percentage
removal |
| 10 | 500 | 97±3 |
| 50 | 500 | 98±3 |
| 100 | 500 | 97±3 |
| 10 | 1000 | 96±3 |
| 50 | 1000 | 95±2 |
| 100 | 1000 | 98±3 |
Table 4: Preconcentration of Ni(II) from DDW and natural tap water samples a.
|
Phase (mg) |
Sample
Volume(mL) |
Ni(II)spiked (pg ml-1) | Preconcentration reagent | Preconcentration
factor |
Ni(II)detecteda (ng ml-1) | Percentage removal |
| 1000 | 2000
Tap water (Saveh) |
20 | 10.0ml of
10.0M HCl |
200 | 5.96±2.7 | 99.0±2.4 |
| 1000 | 2000 | 20 | 10.0ml of | 200 | 5.96±2.0 | 98.9±1.6 |
| DDW | 10.0 M HCl |
a Values are corrected for blank concentration of water samples and based on triplicate analysis.
SEM Investigations
Scanning electron microscopy (SEM) was used to examine the external surface of the fiber before and after modification. As can be seen from Fig. 2, original acrylic fiber comparatively surface (Fig. 2(a)), and with modified fiber (PANF-MMA), obvious change comparing to that of the RAF fiber was observed (Fig.2(b)). It is clear that changes have occurred in the morphology of the fiber
but photographs demonstrated that the surface of PANF-MMA was approximately as smooth, swollen and homogeneous as that of the raw fiber. This can be related to new functional groups that were bigger than (CN) groups.
Scanning electron microscopy (SEM) was so used to examine the morphology of the nano fiber before and after modification. As can be seen from Fig. 3, original acrylic nano fiber comparatively morphology (Fig. 3(a)), and with modified nano fiber (PAN-MMA), obvious change compared to that of the raw fiber was observed (Fig.3 (b)). T modified nano fiber was roundelay as that of raw acrylic nano fiber. This can be related to modification treatment and incorporation of new functional groups into the iber structure.
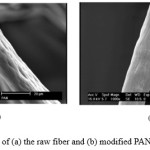 |
Figure 2: SEM image of (a) the raw fiber and (b) modified PAN fiber |
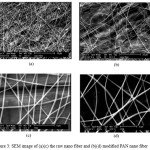 |
Figure 3: SEM image of (a)(c) the raw nano fiber and (b)(d) modified PAN nano fiber |
Selective pre-concentration of Ni(II) from natural water for off-line FAAS
This study was undertaken in order to evaluate the potential application of Modified nano polyacrylonitrile fiber phase for pre-concentration of trace levels of Ni(II) in natural water samples. Drinking tap water was used without prior treatments as an example and compared with double distilled water (DDW) to evaluate and investigate the matrix effect. Both drinking tap water and DDW(2 l) were spiked with 20 pg ml−1 of Ni(II). Several pre-concentration reagents are well known and extensively examined for desorption of the bound metal ions from the surface of the stationary phase and these include mainly, hydrochloric and nitric acid, thiourea HCl [9] as well as ethylenediaminetetraacetic acid [25]. However, some of these reagents are usually characterized by adsorption on the surface of Modified nano polyacrylonitrile fiber which lead to severe change in the nature of packing material as well as non reproducible results if the column used in future applications. The efficiency of metal ion pre-concentration especially Ni(II), by 10.0M HCl acid is found to be high without causing any notable change to the chemical nature of the organic Modified nano polyacrylonitrile fiber. Therefore, 10.0 ml of 10.0M HCl was used for the elution of the adsorbed Ni(II) from the column bed. The pre-concentration factor targeted from this study is 100 as given in Table 4. As the results indicate, the off-line detection results of the eluted and pre-concentrated Ni(II) are very good with a satisfactory pre-concentration factor which can be further increased to 500-fold by simply increasing the water sample volume to 5 l instead of 2 l. Moreover, natural tap water sample was found to give very close results to that reported for DDW sample and this comparison indicates that the matrix effects of the dissolved inorganic and organic matters played an insignificant role in the aimed selective extraction, removal and pre-concentration of Ni(II) by Modified nano polyacrylonitrile fiber phase
References
- R.M. Izatt, J.S. Bradshaw, S.A. Nielsen, J.D. Lamb, J.J. Christensen, D. Sen, Chem. Rev. 1985,85 ,271.
- R.M. Izatt, K. Pawlak, J.S. Bradshaw, R.L. Bruening, Chem. Rev. 1991,91,1721.
- R.M. Izatt, K. Pawlak, J.S. Bradshaw, R.L. Bruening, Chem. Rev. 1995, 95 ,2529.
- A.J. Blake, F. Demartin, F.A. Deviallonova, A. Garau, F. Isaia, V. Lippolis, M. Schroder, G. Verani, J. Chem. Soc., Dalton Trans. 1996,3705.
- M. Arca, A.J. Blake, J. Casab, F. Demartin, F.A. Devillanova, A. Garau, F. Isaia, V. Lippolis, R. Kivekas, V. Muns, M. Schroder, G. Verani, J. Chem. Soc., Dalton Trans. 2001, 1180.
- V. Ghoulipour, S.W. Husain, Acta Chromatographica, 2002,12, 170.
- O. R .Hashemi,. M. Kargar-Razi,; F.Raoufi, A.Moghimi, H.Aghabozorg, M. R.Ganjali, Microchem. J., 2001,69,1.
- N.I. Shcherbinina, G.V. Myasoedova, T.A. Khabazova, E.Y. Shroeva, G.R. Ishmiyarova, I.E. Nikitina, L.N. Bannykh, Zh. Anal. Khim. 1990, 45 ,2137.
- M.M. Gomes-Gomes, M.M. Hidalgo Garcia, M.A. Palacio Corvillo, Analyst 1995,120,1911.
- Unger K., Porous Silica, Elsevier, Amsterdam, 1979.
- Boudreau S.P., Cooper W.T., Anal. Chem. 1989, 61 ,41.
- Kvitek R.J., Evans J.F., Carr P.W., Anal. Chim. Acta,1982, 144 ,93.
- M.L. Bruening, D.M. Mitchell, J.S. Bradshaw, R.M. Izatt, R.L. Bruening, Anal. Chem. 1991, 63 , 21.
- Mahmoud M.E., Talanta ,1997,45 ,309.
- Mahmoud M.E., Soliman E.M., Talata,1997, 44,15.
- Mahmoud M.E., Soliman E.M., Talanta ,1997,44 ,1063.
- Tong A., Akama Y., Tanaka S., Anal. Chim. Acta ,1990,230 ,179.
- Dadler V., Lindoy L.F., Sallin D., Schlaepfer C.W., Aust. J. Chem. 1987, 40 ,1557.
- Mahmoud M.E., in: Proceeding of the 25th FACSS Conference, Austin, TX, USA, 11–15 October, 1998.
- Mahmoud M.E., Anal. Chim. Acta ,1999,398 , 297.
- Leyden D.E., Luttrell G.H., Sloan A.E., DeAngelis N.J., Anal. Chim. Acta ,1976,84 ,97.
- Moghimi, A. Ghiasi R., Abedin A.R. , Ghammamy S. African Journal of Pure and Applied Chemistry 2009,3 (3), pp. 051-059.
- MOGHIMI, A. Oriental Journal of Chemistry 2006,22(3),527.
- Moghimi A., POURSHARIFI M.J. Asian Journal of Chemistry 2008 , 20, No. 7, 5071-5081
- Moghimi A., Tehrani M.S., Waqif Husain S., Material Science Research India 2006,3(1a),27. 26 . Tehrani M.S., Moghimi A., Waqif Husain S., Material Science Research India,2005,3(2),135.
- Mazlum Ardekany M., Ensafi A.A., Naeimi H., Dastanpour A., Shamelli A., Russ. J. Electrochem. 2003,39,269.
- Tahaei P., Abdouss M., Edrissi M., Shoushtari A. M., Zargaran M., Mat.-wiss. u. Werkstofftech. 2008, 39, 839.
- Gode F., E. E, Fuel Process. Technol. 2005, 86, 875.
- Zargaran M., Shoushtari A. M., Abdouss M., J. Appl. Polym. Sci. 2008, 110, 3843.
- Tabarzadi M., Abdouss M., Hasani S. A., Shoushtary A.M., Mat.-wiss. u.Werkstofftech. 2010, 41, No. 4,221
- Shin D. H., Ko Y. G., Choi U. S., Kim W. N., Ind. Eng. Res.2004, 43, 2060.

This work is licensed under a Creative Commons Attribution 4.0 International License.









