Modification of Mesoporous Silica MCM-41 and its Applications- A review
Sana Alahmad
Department of Chemistry, Taibah University, Al-Madinah Al-Munawwarah Saudi Arabia.
Article Received on :
Article Accepted on :
Article Published : 01 Mar 2012
Recent research has looked to develop innovative and powerful novel functionalized mesoporous silica type MCM-41, controlling and tailoring their properties in a very predictable manner to meet the needs of specific applications. Surface functionalization of ordered mesoporous silica has garnered intense interest for use as solid supports due to its large surface area, fast adsorption kinetics and controllable pore size and pore arrangement in comparison to amorphous silica gel. After a brief introduction to mesoporous silica synthesis, the various methods to modify the surface of mesoporous silica materials, as well as applications in catalysis, adsorption, and guest-host chemistry, chemical separations, biosensors and chemosensors, will be discussed.
KEYWORDS:Mesoprous silica; Biosensors; Chemosensors
Download this article as:| Copy the following to cite this article: Alahmad S. Modification of Mesoporous Silica MCM-41 and its Applications- A review. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Alahmad S. Modification of Mesoporous Silica MCM-41 and its Applications- A review. Available from: http://www.orientjchem.org/?p=11778 |
Introduction
Over the past few decades studies in nanoscience and nanotechnology has emerged as one of the most intensively explored fields. The massive quantity of new literature pertaining to nanoscale materials in a wide range of scientific concentrations, such as materials sciences, chemistry, biology, physics, and engineering, has proven the seemingly unlimited potential these materials have to enhance the daily lives of all society.
One of the most promising classes of nanoscale materials are mesoporous silicates. These materials have many unique and advantageous properties such as high mechanical strength, thermal stability, stability under a wide pH range, and highly tunable pore diameters and shapes which may be taken advantage of for size and shape selective organic syntheses under harsh experimental conditions. The covalent attachment of organic functionality by various methods allows for alteration of the hydrophilicity/-phobicity of the mesoporous silica or the addition of moieties which may catalyze organic transformations, used as efficient chromatographic packings for chemical separations, aid in the long-term stability of microelectronic components, or serve as heterogeneous chelates for toxic metal sequestration or sensing. In addition, mesoporous silicas are optically inert and semiconductiong which allows for use in optic and electronic sensing applications for organic compounds such as explosives, volatile organic compounds (VOCs), and biological compounds or byproducts of biological processes.
History and Synthesis
Ordered mesoporous silica materials (OMS) were first synthesized in the early 1990’s 1 by scientists at Mobil Corp. The prominent characteristics of these materials are high surface areas, narrow pore size distribution along with uniform pore size typically in the range of 3nm – 10 nm. Traditionally three mesophases were synthesized collectively known as M41S materials, and are described as mesoporous per the IUPAC nomenclature because of their pore 2 diameters ranging between 2 nm < d < 50 nm. Perhaps the most widely recognized and used OMS is known as MCM-41 (a Mobil code for mesoporous catalytic material), with a regular hexagonal pore structure and ID channels. The other two phases have cubic pore shapes (MCM-48), or lamellar structures (MCM-50).
The synthetic protocol utilizes quaternary alkylammonium salts such as cetyltrimethyl-ammonium bromide (CTABr) in an aqueous solution under basic conditions. Under these conditions the ionic surfactants form spherical micelles which form supramolecular aggregates of micellular rods that act as structure-directing agents (SDAs) or templates, by forming a liquid crystalline phase3. The hydrolysis and condensation of silica precursors, such as tetraethylorthosilicate (TEOS) or tetramethylorthosilicate (TMOS), forms a solid silicate mesostructure around the template (Path 1 in Figure 1) due to electrostatic interactions between the negatively charged silica species and head groups of the surfactant, or by hydrogen bonding interactions. It is also proposed that the mechanism of formation of the liquid crystalline phase is promoted by the introduction of the silicate species. (Path 2 in Figure 1)
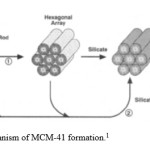 |
Figure 1: Proposed mechanism of MCM-41 formation. Click here to View figure |
Upon formation of the silicate mesostructure, the template is removed by either extraction with solvents such as ethanol or ethanol/diethyl ether mixtures, or high temperature calcinations at 400-550°C. Careful modification of experimental parameters (i.e. alkyl chain length, pH, and surfactant concentration) has yielded mesoporous silicas with hexagonal pore structure, cubic pore shapes, and laminar structures (Figure 2) coined MCM-41, MCM-48, and MCM-50, respectively with varied pore size distributions, pore wall thicknesses and surface areas4.
 |
Figure 2: The X-ray diffraction patterns and proposed structures of MCM-41, MCM-48, and MCM-50 Click here to View figure |
Surface Modification of MCM-41
Various methods have been developed to modify the surface of mesoporous silica materials. Chemical modification of the surface attempts to functionalize the surface with inorganic or organic groups so that the surface properties become better suited for specific applications. Inorganic modification, especially direct doping of the silica surface with metal ions, while important, is outside the scope of this work and therefore will not be discussed.
Introducing organic groups in the readily accessible pores of MCM-41 provide a way of manipulating the chemical and physical properties of these materials without compromising the basic geometry and mechanical strength. Modification of the surface can be achieved chemically (covalent attachment), or physically by adsorption of the functional group 6. The framework of MCM-41 silica material has SiO2 tetrahedra terminating in either siloxane (Si-O-Si) or silanols (Si-OH) on the surface. Both of these groups are reactive towards functionalization, though reaction with silanols is considered to be the main modification pathway 6. Covalent attachment of organic moieties on the surface is termed chemisorption and can be achieved in two major ways co-condensation and post-synthetic modification, or grafting.
Grafting Methods
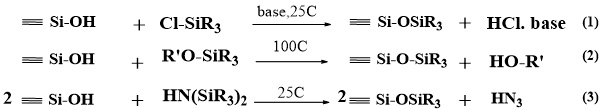
Grafting is a post-synthesis method and it is the most commonly employed functionalization method because of its relative synthetic simplicity and the flexibility in introducing surface groups. This is achieved after surfactant removal and drying of the mature MCM-41. Presence of silanols on the surface in high concentration is an important criterion in this approach. Surface functionalization with organic groups by grafting is most commonly carried out by silylation, which is accomplished by one of the three procedures (Eq. (1) to (3))7. Moreover, esterification is another reaction to carry out surface modification8,9.
As mentioned earlier, high concentration of silanols is important for chemisorptions reaction. The silanols on the surface can be single (isolated or hydrogen bonded), or geminal (two hydroxyls on one silicon) as shown in Figure 3 10. Silylation occurs on all surface groups of the silica including the free or geminal silanols. However, hydrogen-bonded silanol groups are less accessible to the modification because of the formation of hydrophilic networks 11.
The calcination process of making OMS results in loss of most of the surface silanols due to the high temperatures employed. Zhao and Lu demonstrated that the calcinations temperature during the synthesis of MCM-41 materials affects the density of surface silanols12. Ideally a larger concentration of silanol groups is desired because they act as active sites for anchoring organic groups on the surface. At lower calcinations temperatures a large number of the silanols were unavailable for covalent grafting because of hydrogen bonding between them, while at higher temperatures many of the silanols are lost due to condensation reactions. The surface can be rehydrated by boiling the OMS (such as MCM-41) in water followed by azeotropic distillation with benzene or toluene to remove excess water. Secondly, in a more direct method, a stoichiometric amount of water is added based on the surface area of MCM-41. The latter method was developed by scientists at PNNL. By employing just enough water to form a monolayer on the pore surface, a more uniform coat of organosilane is obtained13. This process is termed “self-assembled monolayer on mesoporous supports” (SAMMS). The organosilanes are hydrolyzed to form trishydroxysilanes which undergo self assembly resulting in dense coverage of silica surface14. Adding excess water must be avoided as it may cause the organosilane reagent to form non-surface bound silanol clumps that may block the pores. In other words the silane polymerizes.
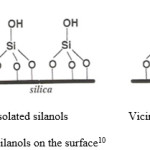 |
Figure 3: Different types of silanols on the surface. Click here to View figure |
Co-condensation Methods
This method was first proposed by Mann et al 15. Co-condensation is a one-pot synthetic method analogous to MCM-41 syntheses where trialkoxyorganosilane species are incorporated into the TEOS (or TMOS) solution and undergo hydrolysis and condensation in the presence of structure directing agents or templates. This method produces mesostructures with organic functionalities covalently attached within the pore walls.
Compared with the grafting method in which the distribution of functional groups often tends to be inhomogeneous, the co-condensation is able to give homogeneously distributed organic groups on the entire inner pore surfaces. Even though the organo-silane may become buried within the walls of the silica matrix and therefore is inaccessible. Since each of the functionalized organo-silane group is in fact a defect in the silica matrix, it adversely affects the stability of the silica structure. This limits the concentration of surface silane groups because the larger the concentration of the silane, the more unstable the silica structure becomes.
There are some readily apparent drawbacks to this methodology, however. One of the major issues is the subsequent removal of templating agent. While calcination is shown to almost completely remove the structure directing agent, such high temperatures will also destroy the covalently attached organic functionality. This leaves solvent extraction as the only possible method. Another advantage of co-condensation over post-synthesis grafting is to control the particle morphology of final mesoporous silicate very easily16,17.
Different surface modification of MCM-41 and their application in the recent years (2000–2012) are summarized in Table 1. The methodologies to characterize the modified MCM-41 include structural analyses such gas sorption studies, powder X-ray diffraction (XRD), and transmission electron microscopy (TEM). Compositional studies such as solid-state NMR, Fourier transform infrared (FTIR), and x-ray photoelectron (XPS) spectroscopic methods, as well as elemental and thermogravimetric analyses will also used.
Table 1: Surface Modification of MCM-41 in the recent years (2000–2012) and their application
|
Modified group |
Application |
Reference |
|
Amino group(NH2 -) |
|
18,19 |
|
Iron complex meso-tetrakis (2,6-dichlorophenyl)porphyrin (FeIIIP) |
Catalyst |
20 |
|
Iminodiacetamide (“IDA-Amide |
Ce3+, Nd3+, Eu3+, Gd3+, and Lu3+ sorption |
21 |
|
Polyacrylonitrile-derived carbon |
Removal of methyl–ethyl ketone |
22 |
|
4-amino-1,8-naphthalimides |
—- |
23 |
|
Mercaptopropyl (MP) and diethylenetriamine (DETA) |
Extraction of mercury (II) |
24 |
|
Lanthanum (III)- diamino |
Phosphate Adsorption |
25 |
|
Calix[4]arene and Calix[4]areneBr |
—– |
26 |
|
β-cyclodextrin |
—— |
27 |
|
Trimethylchlorosilane and methyltrimethoxysilane |
Phenol and hydroquinone adsorption |
28 |
|
Cu diamine ligands (N,N′-dimethyl-1,2-ethanediamine, N,N-dimethyl-1,2-ethanediamine, and N,N-diethyl-1,2-ethanediamine) |
Catalyst. |
29 |
|
Polyethylene oxide |
—– |
30 |
|
Chiral Ru complex |
Catalyst. |
31 |
|
Sulfonated polyimide |
Polymer electrolyte fuel cell |
32 |
|
Glycopyranose |
Borate Adsorption |
33 |
|
Aminopropyl |
Preconcentration of gold and palladium in |
34 |
|
Alkyl chain |
Chromatographic applications |
35 |
|
Transition metal (Ni, Rh and Pd) |
Hydrogen sorption |
36,37 |
|
Aluminium 8-hydroxyquinoline derivatives |
For tuning of the light emitting color |
38 |
|
Platinum group metals (Pd, Pt, Rh, Ir) |
Catalyst. |
39-42 |
|
5-nitro-2-furaldehyde (fural) |
Extraction of uranium(VI) and thorium(IV) |
43 |
|
Ammonium |
Adsorption of anionic dyes |
44 |
|
2-(3-(2-Aminoethylthio)propylthio)ethanamine |
Hg(II) sorption |
45 |
|
3,4,9,10-perylenediimides (PDI) |
—– |
46 |
|
Fe, Mn and Co, tetraphenylporphyrin complexes |
Catalysts |
47 |
|
3-aminopropyl trimethoxysilane |
|
48-50 |
|
Rhodium diphosphine complexes |
Catalyst. |
51-53 |
|
Chiral Mn(III) salen complexes |
Catalyst |
54-56 |
|
Ni2+, Fe3+, Co2+ and Cr3+ |
Catalyst |
57-61 |
|
Al; B |
Catalyst |
62-68 |
|
n-octadecyltrihydridosilane and n-octadecyltrimethoxysilane |
—– |
69 |
|
Octadecyl (C18) |
—– |
70 |
|
5-mercapto-1-methyltetrazole |
Zn2+ sorption |
71 |
|
Sulfonic acid |
Catalyst |
72,73 |
|
Ru- and Fe-based N,N′-bis(2-pyridylmethyl)-N-methyl-(1S,2S)-1,2-cyclohexanediamine complexes |
Catalyst |
74 |
|
Molybdenum(VI) complexes molybdovanadophosphoric acids |
Catalyst |
75-77 |
|
3-phenyl-4-benzoyl-isoxazol-5-one (HPBI), |
Extraction of Cu(II) |
78 |
|
Nb and Sn |
Oxidation properties |
79 |
|
Thiophene-2-carbaldehyde |
Extraction of palladium(II) |
80 |
|
Au |
Catalyst |
81 |
|
Salicylaldehyde |
Preconcentration of uranium |
82 |
|
Al, Zr, W, B, or P |
Catalyst |
83,84 |
|
Phosphorus P2O5 |
Catalyst |
85 |
|
Copper–Schiff base complex |
Catalyst |
86 |
|
Titanium oxide |
Sorption of CO2 |
87 |
|
N-methylglucamine |
Preconcentration of boron |
88 |
|
Ru/carboxyphosphine |
Catalyst |
89 |
|
Vanadium |
Catalyst |
90-92 |
|
polyethylenimine (PEI). |
CO2 sorption |
93 |
|
Uranyl groups (UO22+) |
Catalyst |
94,95 |
|
Mn(II) |
Catalyst |
96,97 |
|
Tungstophosphoric acid |
Catalyst |
65,98 |
Applications of Mesoporous Silicas
Mesoporous silicas offer many favorable properties with potential for use in a vast array of applications. The major applications to be discussed includes: catalysis, chemo- and biosensing and chemical separations.
Catalysis
Mesoporous silica provides a unique platform for catalytic processes with several advantages over traditional homogeneous catalysis. Highly tunable pore size distributions and arrangements (i.e. hexagonal, lamellar, cubic, spherical etc.) allows forsize and shape selective catalysis. High surface areas and site isolation techniques allow for the covalent attachment of significant quantities of non-interacting catalytic sites. Homogenous materials such as organometallic compounds can be expensive to synthesize and require time consuming and/or exotic recovery methodologies. Other catalytic reagents such as acids/bases in acid or base catalyzed reactions may limit the choice of solvent systems and require extensive work-up procedures. Unlike homogenous catalysts, however, the silica mesostructure may be altered (i.e. hydrophilicity/-phobicity, size and shape) for stability in a wide range of reaction media, temperatures and pressures and may be easily separated from the reaction mixture by vacuum or gravity filtration and recycled for multiple uses with little or no loss of catalytic activity. To date there have been a plethora of organic, inorganic, and organometallic catalysts immobilized on highly ordered mesoporous silica. For example 3-aminopropyl functionalized mesoporous silicates have been shown an efficient heterogeneous base catalyst for nitroaldol and Michael addition 99,100.
Mesoporous Chemosensors
One of the many significant potential applications of mesoporous silica is their use as platforms for chemosensing applications. Metal ion detection using fluorescence and UV-Vis spectroscopic measurements has been studied extensively to produce functionalized mesoporous silicates with high metal ion specificities 101. Mesoporous silica has also proven an efficient scaffold for volatile organic compound (VOC) and explosive chemosensing applications102. In addition to the fabrication of sensors for metal ions and VOCs, many studies have utilized mesoporous silica as a scaffold in gas sensing applications 103.
Mesoporous Biosensors
There have been intensive studies using mesoporous silica as a scaffold for various biological sensing techniques. Large surface areas and pore diameters allow for the incorporation of biomolecules toward the fabrication of bioelectrochemical sensors 104,105. Surface functionalization with target specific compounds and the high biocompatibility of mesoporous silica allows for imaging biological processes. To date, there have been numerous studies dedicated toward the immobilization of biological species.
Chemical Separations
Mesoporous silicates have received considerable interest in chemical separations and as adsorbents due to their large surface areas, easily tunable pore size distributions, mechanical and thermal stabilities, and resistance to decomposition in a wide pH range. Furthermore, the ability to alter the hydrophilicity/-phobicity, via addition of non-polar organic groups (i.e. alkyl, vinyl, or phenyl substituents), or the addition of reactive functional groups (such as amines, thiols or specialized chelating agents) allows for size, shape or functionality specific reactivity. These properties have been taken advantage of in the separation/adsorption of heavy metals, organic compounds with high target material specificities and efficiencies. For instance, N-(2-aminoethyl)dithiocarbamate functionalized MCM-41 has been prepared by Stathi et al106.These materials are promising heavy metal adsorbents due to the presence of the effective dithiocarbamate groups and the low pH value (3.2) of the point of zero charge.
As mentioned, functionalized mesoporous silicates have also proven very effective as chromatographic packings for the separation of organic compounds. In a study by Yasmin and Müller 35 various n-butyl and n-octyl functionalized MCM-41 spheres were tested for separation efficiency of NIST SRM 870, which consists of five organic components (uracil, toluene, ethylbenzene, quinizarin and amitriptyline).
Chiral compound separations have also successfully been performed. Zhu et al 107 efficiently separated enantiomeric mixtures of R/S-1,10-bi-2-naphthol using mesoporous organic–inorganic spheres with covalently bridged trans-(1R,2R)-bis-(ureido)-cyclohexane synthesized by cocondensation of N,N’-bis-[(triethoxysilyl)propyl]-trans- (1R,2R)-bis-(ureido)- cyclohexane and 1,2-bis(trimethoxysilyl)ethane.
Conclusion
The synthesis, characterization, and application of novel porous materials have been strongly encouraged due to their wide range of applications. Functionalization of the surface of these mesoporous materials with organic or inorganic functional groups leads to new physical and chemical properties. These modified materials can be used in a variety of applications such as catalysis, adsorption, and separation as chromatographic column packing.
Reference
- Beck, J. S., Vartuli, J. C., Roth, W. J., Leonowicz, M. E., Kresge, C. T., Schmitt, K. D., Chu, C. T. W., Olson, D. H., Sheppard, E. W., McCullen, S. B., Higgins, J. B., Schlenker, J. L., Journal of the American Chemical Society, 114, 10834 (1992).
- McCusker, L. B., Liebau, F., Engelhardt, G., Pure and Applied Chemistry, 73, 381-394(2001).
- Sayari, A. ,Chemistry of Materials, 8, 1840 (1996).
- Stucky, G. D., Monnier, A., Schüth, F., Huo, Q., Margolese, D., Kumar, D., Krishnamurty, M., Petroff, P., Firouzi, A., Janicke, M., Chmelka, B. F., Molecular Crystals and Liquid Crystals Science and Technology. Section A. Molecular Crystals and Liquid Crystals, 240, 187 (1994).
- Barton, T. J., Bull, L. M., Klemperer, W. G., Loy, D. A., McEnaney, B., Misono, M., Monson, P. A., Pez, G., Schere, G. W., Vartuli, J. C., Yaghi, O. M., Chemistry of Materials, 11, 2633 (1999).
- Price, P. M., Clark, J. H., Macquarrie, D. J., Journal of the Chemical Society, Dalton Transactions, 101 (2000).
- Anwander, R., Palm, C., Stelzer, J., Groeger, O., Engelhardt, G., 117, 135 (1998).
- Beck, J. S., Calabro, D. C., McCullen, S. B., Pelrine, B. P., Schmitt, K. D., Vartuli, J. C. Mobil Oil Corp., USA (1992).
- Kimura, T., Saeki, S., Sugahara, Y., Kuroda, K. A., Langmuir, 15, 2794 (1999).
- Jal, P. K., Patel, S.,; Mishra, B. K., Talanta, 62, 1005 (2004).
- Zhao, X. S., Lu, G. Q., J.Phys. Chem. B., 102, 1556 (1998).
- Zhao, X. S., Lu, G. Q.; Whittaker, A. K.; Millar, G. J., Zhu, H. Y., Journal of Physical Chemistry B, 101, 6525 (1997).
- Stein, A., Melde, B. J., Schroden, R. C., Advanced Materials, 12, 1403(2000).
- Feng, X., Fryxell, G. E., Wang, L. Q., Kim, A. Y., Liu, J., Kemner, K. M., Science, 276, 923 (1997).
- Burkett, S. L., Sims, S. D., Mann, S., Chemical Communications, 1367 (1996).
- Ozin, G. A., Chemical Communications, 419 (2000).
- Yang, H., Coombs, N., Ozin, G. A., Nature, 386, 692 (1997).
- Marília, R. M., Delphine, P., Gleiciani, Q. S., Philip, L. L., Célia, M. R., Microporous and Mesoporous Materials, 143, 174 (2011).
- Aghdas, H., Habibollah, Y., Zahra, M., Chemical Engineering Journal, 153, 70 (2009).
- Molinari, A., Maldotti, A., Bratovcic, A., Magnacca, G., Catalysis Today, 161, 64 (2011).
- Glen, E. F., Wilaiwan, C., Ryan, D. R., Inorganic Chemistry Communications, 14, 971 (2011).
- Janus, R., Kuśtrowski, P., Dudek, B., Piwowarska, Z., Kochanowski, A., Michalik, M., Cool, P., Microporous and Mesoporous Materials, 145, 65 (2011).
- de Jesus Trindade, F., Queiruga Rey, J. F., Brochsztain, S., Dyes and Pigments, 89, 97(2011).
- Idris, S. A., Harvey, S. R., Gibson, L. T. Journal of Hazardous Materials, 193, 171(2011).
- Zhang, J., Shen, Z., Shan, W., Mei, Z., Wang, W. Journal of Hazardous Materials, 186, 76(2011).
- Li, Y.-J., Yan, B., Wang, L. Journal of Solid State Chemistry, 184, 2571(2011).
- Nan, Z., Xue, X., Hou, W., Yan, X., Han, S. Journal of Solid State Chemistry, 180, 780(2007).
- Fu, J., He, Q., Wang, R., Liu, B., Hu, B. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 375, 136(2011).
- Jana, S., Bhunia, S., Dutta, B., Koner, S. Applied Catalysis A: General, 392, 225(2011).
- Jomekian, A., Mansoori, S. A. A.; Monirimanesh, N. Desalination, 276, 239(2011).
- Lou, L.-L., Dong, Y., Yu, K., Jiang, S., Song, Y., Cao, S., Liu, S. Journal of Molecular Catalysis A: Chemical, 333, 20(2010).
- Okamoto, K.-i., Yaguchi, K., Yamamoto, H., Chen, K., Endo, N., Higa, M., Kita, H. Journal of Power Sources, 195, 5856(2010).
- Fried, D. I., Schlossbauer, A., Bein, T. Microporous and Mesoporous Materials, 147, 5(2012).
- Ebrahimzadeh, H., Tavassoli, N., Amini, M. M., Fazaeli, Y., Abedi, H. Talanta, 81, 1183(2010).
- Yasmin, T., Müller, K. Journal of Chromatography A, 1217, 3362(2010).
- Prasanth, K. P., Raj, M. C., Bajaj, H. C., Kim, T. H., Jasra, R. V. International Journal of Hydrogen Energy, 35, 2351(2010).
- Boutros, M., Launay, F., Nowicki, A., Onfroy, T., Herledan-Semmer, V., Roucoux, A., Gédéon, A. Journal of Molecular Catalysis A: Chemical, 259, 91(2006).
- Fazaeli, Y., Amini, M. M., Mohajerani, E., Sharbatdaran, M., Torabi, N. Journal of Colloid and Interface Science, 346, 384(2010).
- Ravat, V., Mantri, D. B., Selvam, P., Aghalayam, P. Journal of Molecular Catalysis A: Chemical, 314, 49(2009).
- Mastalir, Á., Rác, B., Király, Z., Tasi, G., Molnár, Á. Catalysis Communications, 9, 762(2008).
- Mastalir, Á., Rác, B., Király, Z., Molnár, Á. Journal of Molecular Catalysis A: Chemical, 264, 170(2007).
- Papp, A., Molnár, Á., Mastalir, Á. Applied Catalysis A: General, 289, 256(2005).
- Yousefi, S. R., Ahmadi, S. J., Shemirani, F., Jamali, M. R., Salavati-Niasari, M. Talanta, 80, 212(2009).
- Qin, Q., Ma, J., Liu, K. Journal of Hazardous Materials, 162, 133(2009).
- Puanngam, M., Unob, F. Journal of Hazardous Materials, 154, 578(2008).
- Trindade, F. J., Fernandes, G. J. T., Araújo, A. S., Fernandes Jr, V. J., Silva, B. P. G., Nagayasu, R. Y., Politi, M. J., Castro, F. L., Brochsztain, S. Microporous and Mesoporous Materials, 113, 463(2008).
- Costa, A. A., Ghesti, G. F., de Macedo, J. L., Braga, V. S., Santos, M. M., Dias, J. A., Dias, S. C. L. Journal of Molecular Catalysis A: Chemical, 282, 149(2008).
- Anbia, M., Lashgari, M. Chemical Engineering Journal, 150, 555(2009).
- Kim, M. L., Stripeikis, J. D., Tudino, M. B. Talanta, 77, 1068(2009).
- Zeleňák, V., Badaničová, M., Halamová, D., Čejka, J., Zukal, A., Murafa, N., Goerigk, G. Chemical Engineering Journal, 144, 336(2008).
- Crosman, A., Hoelderich, W. F. Journal of Catalysis, 265, 229(2009).
- Crosman, A., Hoelderich, W. F. Catalysis Today, 121, 130(2007).
- Wagner, H. H., Hausmann, H., Hölderich, W. F. Journal of Catalysis, 203, 150(2001).
- Yu, K., Gu, Z., Ji, R., Lou, L.-L., Liu, S. Tetrahedron, 65, 305(2009).
- Yu, K., Gu, Z., Ji, R., Lou, L.-L., Ding, F., Zhang, C., Liu, S., Journal of Catalysis, 252, 312(2007).
- Lou, L.-L., Yu, K., Ding, F., Peng, X., Dong, M., Zhang, C., Liu, S., Journal of Catalysis, 249, 102(2007).
- Wu, C., Gao, Q., Hu, J., Chen, Z., Shi, W., Microporous and Mesoporous Materials, 117, 165(2009).
- Somanathan, T., Pandurangan, A. Applied Surface Science, 254, 5643(2008).
- Shylesh, S., Samuel, P. P., Singh, A. P. Applied Catalysis A: General, 318, 128(2007).
- Choi, J.-S., Yoon, S.-S., Jang, S.-H., Ahn, W.-S. Catalysis Today, 111, 280(2006).
- Vrålstad, T., Øye, G.; Rønning, M., Glomm, W. R., Stöcker, M., Sjöblom, J. Microporous and Mesoporous Materials, 80, 291(2005).
- Adjdir, M., Ali-Dahmane, T., Weidler, P. G. Comptes Rendus Chimie, 12, 793(2009).
- Habib, S., Launay, F., Laforge, S., Comparot, J.-D., Faust, A.-C., Millot, Y., Onfroy, T., Montouillout, V., Magnoux, P., Paillaud, J.-L., Gédéon, A. Applied Catalysis A: General, 344, 61(2008).
- Ramachandran, S., Ha, J.-H., Kim, D. K. Catalysis Communications, 8, 1934(2007).
- Nandhini, K. U., Arabindoo, B., Palanichamy, M., Murugesan, V. Journal of Molecular Catalysis A: Chemical, 243, 183(2006).
- Prokešová, P., Žilková, N., Mintova, S., Bein, T., Čejka, J. Applied Catalysis A: General, 281, 85(2005).
- Udayakumar, S., Pandurangan, A., Sinha, P. K. Applied Catalysis A: General, 272, 267(2004).
- Selvaraj, M., Pandurangan, A., Seshadri, K. S., Sinha, P. K., Krishnasamy, V., Lal, K. B. Journal of Molecular Catalysis A: Chemical, 186, 173(2002).
- Kailasam, K., Fels, A., Müller, K. Microporous and Mesoporous Materials, 117, 136(2009).
- Kailasam, K., Müller, K. Journal of Chromatography A, 1191, 125(2008).
- Pérez-Quintanilla, D., Sánchez, A., del Hierro, I., Fajardo, M., Sierra, I. Journal of Colloid and Interface Science, 313, 551(2007).
- Shylesh, S., Samuel, P. P., Srilakshmi, C., Parischa, R.; Singh, A. P. Journal of Molecular Catalysis A: Chemical, 274, 153(2007).
- Sheng, X., Gao, J., Han, L., Jia, Y., Sheng, W. Microporous and Mesoporous Materials, 143, 73(2011).
- Soundiressane, T., Selvakumar, S., Ménage, S., Hamelin, O., Fontecave, M., Singh, A. P. Journal of Molecular Catalysis A: Chemical, 270, 132 (2007).
- Bordoloi, A., Lefebvre, F., Halligudi, S. B. Journal of Catalysis, 247, 166(2007).
- Masteri-Farahani, M., Farzaneh, F., Ghandi, M. Catalysis Communications, 8, 6(2007).
- Célestin Bakala, P., Briot, E., Salles, L., Brégeault, J.-M. Applied Catalysis A: General, 300, 91(2006).
- Miloudi, H., Boos, A., Bouazza, D., Ali-Dahmane, T., Tayeb, A., Goetz-Grandmont, G., Bengueddach, A. Materials Research Bulletin, 42, 769(2007).
- Nowak, I., Feliczak, A., Nekoksová, I., Čejka, J. Applied Catalysis A: General, 321, 40(2007).
- Jamali, M. R., Assadi, Y., Shemirani, F., Salavati-Niasari, M. Talanta, 71, 1524(2007).
- Sobczak, I., Kusior, A., Grams, J., Ziolek, M. Journal of Catalysis, 245, 259(2007).
- Jamali, M. R., Assadi, Y., Shemirani, F., Hosseini, M. R. M.; Kozani, R. R.; Masteri-Farahani, M., Salavati-Niasari, M. Analytica Chimica Acta, 579, 68(2006).
- Kucherov, A. V., Shigapov, A. N., Ivanov, A. V., Kucherova, T. N., Kustov, L. M. Catalysis Today, 110, 330(2005).
- Chen, L. F., Wang, J. A., Noreña, L. E., Aguilar, J., Navarrete, J., Salas, P., Montoya, J. A., Del Ángel, P. Journal of Solid State Chemistry, 180, 2958(2007).
- Herrera, J. M., Reyes, J., Roquero, P., Klimova, T. Microporous and Mesoporous Materials, 83, 283(2005).
- Singh, U. G., Williams, R. T., Hallam, K. R., Allen, G. C. Journal of Solid State Chemistry, 178, 3405.( 2005)
- Singh, U. G., Williams, R. T., Hallam, K. R., Allen, G. C. Solid State Sciences, 7, 1104(2005).
- Kaftan, Ö., Açıkel, M., Eroğlu, A. E., Shahwan, T., Artok, L., Ni, C. Analytica Chimica Acta, 547, 31(2005).
- Štěpnička, P., Demel, J., Čejka, J. Journal of Molecular Catalysis A: Chemical, 224, 161(2004).
- Selvam, P., Dapurkar, S. E. Applied Catalysis A: General, 276, 257(2004).
- Shylesh, S., Singh, A. P. Journal of Catalysis, 228, 333(2004).
- Sarkadi-Pribóczki, E., Tsoncheva, T. Catalysis Communications, 10, 1216(2009).
- Xu, X.; Song, C., Andrésen, J. M., Miller, B. G., Scaroni, A. W. Microporous and Mesoporous Materials, 62, 29(2003).
- Kumar, D., Varma, S., Dey, G. K., Gupta, N. M. Microporous and Mesoporous Materials, 73, 181(2004).
- Štamberg, K., Venkatesan, K. A., Vasudeva Rao, P. R. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 221, 149(2003).
- Vetrivel, S., Pandurangan, A. Journal of Molecular Catalysis A: Chemical, 217, 165(2004).
- Vetrivel, S., Pandurangan, A. Applied Catalysis A: General, 264, 243(2004).
- Jentys, A., Schießer, W., Vinek, H. Catalysis Today, 59, 313(2000)
- Sharma, K. K., Asefa, T. Angewandte Chemie – International Edition, 46, 2879(2007).
- Macquarrie, D. J., Maggi, R., Mazzacani, A., Sartori, G., Sartorio, R. Applied Catalysis A: General, 246, 183(2003).
- Gao, L., Wang, Y., Wang, J., Huang, L., Shi, L., Fan, X., Zou, Z., Yu, T., Zhu, M., Li, Z. Inorganic Chemistry, 45, 6844(2006).
- Sasahara, T., Kido, A., Ishihara, H., Sunayama, T., Egashira, M. Sensors and Actuators, B: Chemical, 108, 478(2005).
- Yuliarto, B., Honma, I.,Katsumura, Y., Zhou, H. Sensors and Actuators, B: Chemical, 114, 109(2006).
- Xu, X., Lu, P., Zhou, Y., Zhao, Z., Guo, M. Materials Science and Engineering C, 29, 2160(2009).
- Chouyyok, W., Panpranot, J., Thanachayanant, C., Prichanont, S. Journal of Molecular Catalysis B: Enzymatic, 56, 246(2009).
- Stathi, P., Dimos, K., Karakassides, M. A., Deligiannakis, Y. Journal of Colloid and Interface Science, 343, 374(2010).
- Zhu, G., Zhong, H., Yang, Q., Li, C. Microporous and Mesoporous Materials, 116, 36(2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









