Kinetic Study of PMMA Synthesis by Batch Emulsion Polymerization
Saurabh , Sushant Upadhyaya, Rajeev Kr. Dohare, Madhu Agarwal and Alok Gupta
Department of Chemical Engineering, Malaviya National Institute of Technology, Jaipur - 302017 (India).
Polymethyl Methacrylate (PMMA) is a versatile transparent thermoplastic polymer. It has fairly good impact resistant and excellent biocompatibility; therefore PMMA used commercially where high pressure tolerance and good transparencies both are required simultaneously. In this study kinetics of polymerization process was investigated by analysis of degree of conversion, rate of polymerization with respect to time. In this report it has confirmed that use of 2.5 gm. Potassium Persulphate (KPS) as initiator give fairly good conversion and satisfactory rate of polymerization.
KEYWORDS:Emulsion polymerization; PMMA; Surfactant; Initiator; Emulsifier; Critical Micelles Concentration (CMC)
Download this article as:| Copy the following to cite this article: Saurabh S, Upadhyaya S, Dohare R. K, Agarwal M, Gupta A. Kinetic Study of PMMA Synthesis by Batch Emulsion Polymerization. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Saurabh S, Upadhyaya S, Dohare R. K, Agarwal M, Gupta A. Kinetic Study of PMMA Synthesis by Batch Emulsion Polymerization. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24052 |
Introduction
Polymethyl Methacrylate (PMMA) has been used in formation of in-house and deep sea aquarium, bombardier nose, motorcycle helmet visors etc. In medical technologies it has been used for bone implants, denture formation, cosmetic surgery etc. Initially Fittig and Poul in1877 discovered the polymerization processes that convert Methyl Methacrylate into PMMA (Lovell 1997). In 1933 Rohm and Haas Company commercially introduced PMMA into market under the trademark Plexiglass. By emulsion polymerization a variety of synthetic material like paints, toners, adhesive, coatings etc. are being commercially produce by various industries.
Emulsion polymerization involves conversion of emulsified monomer into a stable dispersion of polymer particles by free-radical propagation mechanism. It contains the emulsification of monomers in a continuous aqueous phase, stabilization of the initial droplets and final latex particles by a surfactant. Surfactants have a large influence on the latex product properties, e.g. particle size distribution, molecular weight and rheological properties.
In this paper the synthesis and kinetic study of PMMA was carried out in batch emulsion polymerization reactor using Potassium Persulphate (KPS) as initiator and Sodium Oleate as surfactant and distilled water as medium. Water soluble initiator reacts with monomer into micelles and MMA turned into polymerized PMMA. Micelles formation occurs when the level of surfactant is beyond its critical micelles concentration (CMC). Free radicals are produced in the aqueous phase by initiator decomposition and captured by the micelles swollen with monomer. The polymerization begins in these micelles. For batch emulsion polymerization processes Smith and Ewart(1948) derived the relation for rate of polymerization and no. of particle[2].
Nα Rp α CI00.4 (CE0-CCMC) 0.6 C 0 M0
For emulsion polymerization in CSTR Degraff and Poehlein(1971) derived a relation for no. of particle and rate of polymerization in steady state as follows[3].
N α Rp α CI00 (CE0-CCMC) C 0 M0τ-2/3
This shows that particle distribution depends on residence time in case of CSTR emulsion polymerization. It was assumed that rate of radical absorption into micelles and existing particles is directly proportional to their surface area.
The objective of current study is synthesis of PMMA by batch emulsion polymerization. The kinetics of process was investigated by study of conversion, rate of polymerization etc. with respect to time in a controlled stirred tank batch reactor system.
Experimental
Materials
Feed composition had the following ingredients- Methyl Methacrylate monomer was used as a main ingredient (Central Drug House P Ltd.), KPS as initiator (laboratory reagent, MERCK), Surfactant (Sodium Oleate, laboratory reagent, MERCK), Solvent(ion-exchanged double distilled water), Solubilizer (Benzene),Terminator (methanol, Central drug house (P) LTD, batch No-04014 and product No-029192.) etc was used.
Experimental Set Up
The experimental apparatus consisted of a 2.14 litre cylindrical vessel made of stainless steel sheet with inside diameter of 170mm and height of 190mm. Stainless-steel stirred-tank reactors provided with a high speed mixer .It is equipped with four baffles and with external jackets for heating and cooling Proper arrangements are provided for the control of reaction temperature and the RPM of the mixer. Vacuum pump and Nitrogen cylinder were installed for evacuation and bubbling of N2 within reactor respectively.
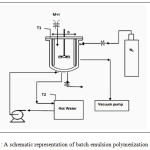 |
Figure 1: A schematic representation of batch emulsion polymerization reactor. Click here to View figure |
Preparation of feed charge
Following composition of ingredients were prepared, which have proportions compatible with Flory composition [4].In 220 ml. of ion exchanged double distilled water, 200 ml. of monomer Methyl Methaacrylate was added. The weight of initiator (KPS) and Surfactant (Sodium Oleate) were kept at range of 2.5 gm and 2.0 gm respectively
Experimental Procedure
Emulsion polymerization was carried out in stainless-steel stirred-tank reactors with a mechanical stirring at 700 rpm to 750 rpm, under constant nitrogen supply at 3 LPM. Temperature of reactor system was kept constant at 70 0C ± 1 0C .
Pre-weighed emulsifier and water were charged in the reactor and the addition of MMA monomer was followed. Evacuation of reactor was done with the help of vacuum pump. Evacuation and constant N2 supply is mandatory to ensure absence of oxygen into reactor. Agitation was start at rate of 700-750rpm and simultaneous bubbled the N2 AT 3 LPM into reaction mixture. After exactly one hour of agitation the aqueous KPS solution was then added from the top rear of set-up under an atmosphere of N2 , and the polymerization was initiated.
During the polymerization, aliquots of the reaction mixture were withdrawn from the reactor at different time interval to examine the kinetics of reaction.
Experimental Data
Polymer was collected and addition of Methanol with Hydroquinone was done to terminate the reaction and precipitate the polymer. Polymer was dried in oven at 800C.The weight of dry polymer and degree of conversion (X) is tabulated in table 1. Result of batch emulsion polymerization has been estimated through conversion of monomer with respect to time analysis. During polymerization monomer is converted into polymerized form so as polymerization proceed forward with time, monomer concentration reduces.
Table 1
| S.N. | Time (min.) | Sample taken | Wt. of dry polymer (gm.) | Conversion (X) | |
| ml. | gm. | ||||
|
1. |
0 |
20 |
19.17 |
0 |
0 |
|
2. |
20 |
20 |
21.12 |
2.706 |
0.3391 |
|
3. |
40 |
20 |
21.64 |
4.413 |
0.5397 |
|
4. |
60 |
20 |
21.89 |
5.144 |
0.622 |
|
5. |
80 |
20 |
22.01 |
5.602 |
0.6736 |
|
6. |
100 |
20 |
22.11 |
5.816 |
0.6962 |
|
7. |
120 |
20 |
22.09 |
5.86 |
0.7021 |
|
8. |
140 |
20 |
22.11 |
5.98 |
0.7158 |
|
9. |
160 |
20 |
22.00 |
6.038 |
0.7264 |
|
10. |
180 |
20 |
22.00 |
6.038 |
0.7264 |
Rate of polymerization has been calculated and tabulated in table 2.2. It shows that rate of polymerization was maximum as 17.34 (g/l.min) at 160 minute and after this the rate of polymerization decreases as the reaction proceed forward with time and becomes 16.047 (g/l.min) for 200 minute.
Table 2
| Time(min.) | dX/dt | Rate of polymerization(g/l.min) |
|
0 |
0.021 |
7.488 |
|
20 |
0.022104 |
7.8822 |
|
40 |
0.025032 |
8.92641 |
|
60 |
0.029208 |
10.41 |
|
80 |
0.034056 |
12.144 |
|
100 |
0.039 |
13.90 |
|
120 |
0.04346 |
15.49 |
|
140 |
0.046872 |
16.714 |
|
160 |
0.04864 |
17.34 |
|
180 |
0.04821 |
17.19 |
|
200 |
0.045 |
16.047 |
Results and Discussion
The synthesis of Poly methyl Methacrylate was done by batch emulsion polymerization with Methyl Methacrylate as the monomer potassium per-sulfate as the initiator, sodium Oleate as the emulsifier and distilled water as a medium. The kinetics, degree of conversion, rate of polymerization of emulsion polymerization was studied. Emulsion polymerization is exothermic reaction in nature. Temperature can be maintained by external medium (cooling/heating). Control valve are also used to control the flow rate of hot/cold water.
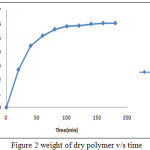 |
Figure 2: weight of dry polymer v/s time |
Weight of dry polymer v/s time: It shows that in emulsion polymerization process, initially weight of polymer formation increases and after 120 minute agitation, polymer formed was 5.98 gm. The weight of polymer formed become nearly constant as the reaction proceeds forward with time.
Degree of conversion V/s time:It shows the conversion of MMA monomer into PMMA as a function of time. Degree of conversion X increases with time T initially and then equilibrates between 0.7158 to 0.7264 as shown in table. Initially increase in the conversion rate observed due to Trommsdorrf effect. This phenomenon occurs due to localized increase in viscosity, it causes the motion of radical restricted to approach each other for the termination, and hence it decreases the termination rate.
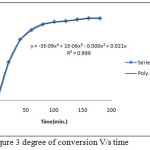 |
Figure 3: degree of conversion V/s time Click here to View figure |
Rate of polymerization V/s time
It has been reported that faster initiation rate leads to narrower particle size distribution.[1] Increasing the radical concentration in emulsion polymerization leads to decrease in size of polymer molecule. The concentration of Polymethyl Methacrylate increases with time as reaction proceeds in forward direction. Agitation speed affect the kinetics and degree of conversion of MMA monomer into PMMA.[5]. The conversion (X) seems to be strongly dependent on the hydrodynamic characteristics of the reactor and the polymerization conditions. Figure 4 shows that initially rate of polymerization for particular time interval 20 min increases.
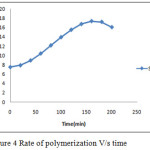 |
Figure 4: Rate of polymerization V/s time |
By Smith Ewart theory of emulsion polymerization, initially rate of polymerization increases due to increasing the availability of free radical and monomer into the micelle. In figure 5 it shows that after 160 minute reaction the rate decreases as the reaction proceeds further. The decrement in rate is due to depletion of free radical particle as termination of free radical starts
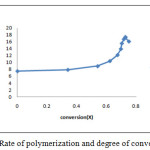 |
Figure 5: Rate of polymerization and degree of conversion |
Figure 5 shows that rate of polymerization was increased very drastically in between degree of conversion having 0.5 to 0.7 ranges. At 0.7 and above degree of conversion, rate of polymerization decreases due to increase in free radical termination and decrease in monomer concentration during polymerization.
Conclusion
In the present work, the synthesis of PMMA has been successfully done. The kinetics of Methyl Methacrylate emulsion polymerization was studied at 700C using Sodium Oleate as surfactant and KPS as initiator. Corresponding graphs were obtained for conversion, rate of polymerization, with time. In this way complete analysis of batch emulsion polymerization was done. With the successful synthesis of PMMA from emulsion polymerization we got better experimental yields in terms of degree of conversion i.e. 0.7284. This experimental result shows fairly good conversion and satisfactory polymerization rate as compared to other polymerization techniques [4] and thus it is suggested that this technique should be extended for the commercially preparation of other polymers as well.
References
- Kemmere,Maria F “Batch emulsion polymerization” . Proefschrift. – ISBN 90-386-2611-8 Eindhoven : Technische Universiteit Eindhoven1999,pp-42
- Chern, C.S. [2006], “ Emulsion polymerization mechanisms and kinetics”, Progress PolymerScience. Volume 31, pp. 443-486
- DeGraff, A. W. and Poehlein, G. W., 197 1, “Emulsion polymerization of Styrene in a single continuous stirred-tank reactor”. J. Polym. Sci. Part 2-A 9. 1976.
- Flory, P. J. [1953] “Principles of polymer chemistry”, 1st edition, CornellUniversity press,Ithaca, New York, pp 203-217
- Ramirez Jorge C, Jorge Herrera-Ordonez , Hortensia Maldonado-Textle “ Kinetics of the Styrene emulsion polymerization above cmc. II. Agitation effect on molecular weight” Poymer Bulletin 53,pp 333-337,2005
- Arora P, Jain R, Mathur k, Sharma A, Gupta A. “Synthesis of PMMA by batch emulsion polymerization”. African Journal of Pure and Applied Chemistry Vol.4 (8),pp 152- 157, August 2010.

This work is licensed under a Creative Commons Attribution 4.0 International License.









