Investigation of Adsorption Isotherm of Oxymetholone as a Kind of Steroid Drug by Multi-Wall Carbon Nanotube
Mehdi Vadi1* and Yasin Noshadi2
1Department of Chemistry , Fasa Branch, Islamic Azad University , Fasa, Fars, Iran.
2Department of Chemistry, Firozabad Branch, Islamic Azad University, Firozabad, Iran.
Corresponding author: mahdi_vadi@iaufasa.ac.ir
In this experimental research the adsorption of the isotherm of Oxymetholone as a kind of steroid drug is studied on the multi-wall carbon nanotube (MWCNT) with the spectrophotometer .The amount of adsorption in various concentration was calculated and its related diagram was drawn. The result, which was obtained by Langmuir, Freundlich and Temkin in 296, compared coefficient parameters show that the Temkin model has the most accordance.
KEYWORDS:Adsorption; Isotherm; Oxymetholone; Steroid; Multi-wall carbon nanotube
Download this article as:| Copy the following to cite this article: Vadi M, Noshadi Y. Investigation of Adsorption Isotherm of Oxymetholone as a Kind of Steroid Drug by Multi-Wall Carbon Nanotube. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Vadi M, Noshadi Y. Investigation of Adsorption Isotherm of Oxymetholone as a Kind of Steroid Drug by Multi-Wall Carbon Nanotube. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23836 |
Introduction
Beside the fatness, Phospholipid and Terepene, the lipid essence of plant and animal is consisted of steroids [1]. The structure of these molecules is a 4-ring shaped system. Steroids are the modified Trepene which are made up of non-ring shaped Squalene [2] hydrocarbon in the live parts of the body. The exact way of this wonderful change is so time consuming and complex, but the key steps of them are known. It is due to Conrad Block and John Conford, who awarded the noble prize for their discoveries. Beside the derivation of steroids, which are gained by plant and animals, hundreds of other steroids are synthesized in medical laboratories to reach the new drugs. Oxymetholone is a kind of synthesized drug which is sold in the name of Androl [3] in commercial market. Oxymetholone is an edible steroid, when added to water causes muscles of the body seem larger so quickly and the user becomes so bulky in a short while. Over consumption of this drug causes serious injuries on liver. It causes the liver become toxic and it leads to creation of benign & malignant tumors in the liver. The first carbon allotrope which was discovered in 1985 was named Buckmisterfullerene. This material is also known as Bukyball and Fullerene. But in 1991 a scientist, Soumia Iijima, discovered and produced another structure of carbon so accidently. This kind of structure has such identical properties. At first, he imagined this structure as a kind fullerene which extended in the same direction. But after a while he understood that this sample has different advantages from the fullerenes. So he called that the carbon nanotube, which is of two kinds: The single-wall and multi-wall carbon nanotube [4]. The first one is made up of graphonic sylinder which a diameter of 1 to 2 nanometer. Now if we put 3 or 4 of these nanotubes together, then the multi-wall nonatube will produce. Its external diameter 2 to 25 nanometer and its internal diameter is about 1 to 8 nanometer. The average length of nanometer can be multi micron kind. The discovery of multi-wall nanotube in 1991 resulted in vast survey activities in science about the carbon nano structures [5] and their applications. The main reason of this predicted structure is their small size, low density, high hardness, high strength (the external traction of the multi-wall carbon nano tube is 100 fold of aluminum and their excellent electrical properties. So it is possible that these carbon nanotubes use widely for firmness of materials, the flat monitors in the field distribution, chemical sensor, drug transition and nano technology. The recent studies suggest the usage of carbon nanotube for biologic purposes like crystallization of protein, building the bioreactors and biosensors. These carbon nanotubes are un-soluble in water. So it is necessary to overcome this problem for biological applications. The bonding of functional groups to carbon nanotubes [6] is so useful in medical applications. For example the conjunction of nanotubes to a DNA series can lead to connection of protein in a cancerous cell. The connection of cells to another part of carbone nanotubes can make a «guidance vector» to attack to the cancerous cell and its eradication. The carbon nanotubes especially their multi-layer kind with a definite structures have the capability of producing biosensors.
Experimental part
Material
We used the carbon nanotube with 95% pure degree, production of neutrino Company, the Oxymetalone pill in Alhavi pharmaceutical. The ethanol solvent (96%) which is product of Merck Company was used in this experimental.
Apparatus
In this survey the spectrophotometer (Jenway6505, Model, England) magnetic stirrer (Heidolph, Mr3001 Model), Analytical balance (Sartorius Model), Filter paper (Albet), were used.
Method
At first we solved 50mg of Oxymethanole in 50ml of ethanol and make (1000ppm) solution. After dilution of this solution the consistencies (50-100-150-200) ppm were produced. 10ml of each concentration was taken and 0.01gr of carbon nanotube (MWCNT) was added to each part.
This solution was mixed about 20min with magnetic stirrer. Then amount of concentration was measured with spectrophotometer before and after adding carbon nanotube .
Discussions and results
Study of adsorption isotherms
At first we used this relation [7] for assessing the adsorption capacity:
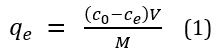
In this equation C0 and Ce are related to the primary concentration and final concentration of Oxymethalone in the solution (mg/L) and V is volume solution (L) and M is mass of adsorpate (g).
Langmuir adsorption model
This model used for monolayer adsorptions. This model formed by the supposition of the equality of energy in the of all the Adsorb sites on this Adsorbent surface. This model is described in this equation which is its liner form [8].
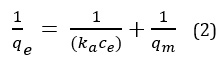
Here qe and ce are the amount of adsorption dissolve at equilibrium (mgg-1) and the equilibrium concentration (mg-1) respectively. Langmuir constants, ka (mgL-1) and qm (mgg-1) are related to the energy of adsorption and the adsorption capacity respectively. One of the characteristic Langmuir equation is Dimensionless of detachment coefficient RL [9] can be gained from (3) relationship.
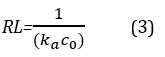
By regarding adsorption with the help of spectrophotometer and the above relation, adsorption Langmuir graph to the 1/qe against 1/ce is plotted(figure 1) and its calculated parameters can be seen in table(1).
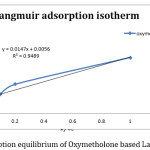 |
Figure 1: Adsorption equilibrium of Oxymetholone based Langmuir isotherm. Click here to View figure |
Temkin model
Temkin isotherm has one factor which shown the interaction between adsorbent and adsorbing particle so vividly. This model was applied in forms, given as equation 4 bellow:
qe=BLNA+BLNCe (4)
B=RT/b (5)
By plotting qe against lnce gave the constants, A And B which are the Temkin isotherm constants (l/mg)[10] and the Temkin constant related to heat of adsorption (jmol-1)respectively is the gas constant (8.314mol/k)B is also a Temkin isotherm constant while is the absolute temperature in Kelvin.
By regarding adsorption with the help of spectrophotometer and the above relation, adsorption Temkin graph to the qe against lnce is plotted(figure 2) and its calculated parameters can be seen in table(1).
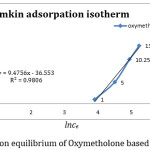 |
Figure 2: Adsorption equilibrium of Oxymetholone based Temkin isotherm. Click here to View figure |
Freundlich model
It is an experimental equation which in used more in adsorption metallic ions on Heterogeneous surface with the multilayer adsorption. The amount of dissolved solvent will increase infinitely with increase of consecration. The linear Freundlich model is expressed as shown in equation 3;
Lnqe=lnkf+ lnce (6)
qe and ce is the amount of dissolve adsorption at equilibrium (mg/g)and equilibrium concentration(mg/L)respectively .kf and are factor which affect the adsorption process(adsorption capacity and intensity respectively).
By regarding adsorption[11] with the help of spectrophotometer and the above relation, adsorption Freundlich graph to the lnce against lnqe is plotted(figure 3) and its calculated parameters can be seen in table(1).
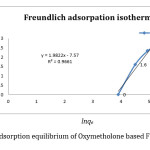 |
Figure 3: Adsorption equilibrium of Oxymetholone based Freundlich isotherm. Click here to View figure |
Table 1: parameters and correlation coefficient of Langmuire ,Temkin and Freundlich model for Oxymetholone .
|
Langmuir model |
Temkin model |
Freundlich model |
isotherm |
|
R2 Ka(L/mg) q(mg/g) RL |
R2 A(L/mg) b B
|
n kf(mg/g) R2 |
parameters |
|
0.948 0.357 200 0.73 |
0.980 0.24 9.47 10.31 |
0.5 5.15×10-4 0 .966 |
values |
Investigation the results of table 1
The result of isotherms show the R2value range obtained for the temkin model is high compared to those of both the Freunlich and Langmuir models. It therefore stands for this adsorption studies, the Temkin model is most suitable and that applicability flows the order; Temkin>Freundlich>Langmuir adsorption model. n Constant is adsorption intensity .if the value of n becomes between 0.1 to1 ,then it shows a suitable flowing from freundlicc isotherm .its shown that the adsorption is desirable. If in Langmuir isotherm, [12] RL>1 then the kind of Langmuir model is undesirable for the experiment. For RL=1 there exist a liner process, and if 1>RL>0, the process is desirable. If RL=0 then the isotherm is irreversible.
Conclusion
The results of this survey show the correlation coefficient of Temkin isotherm equation has the best accordance and its adsorption energy is high. The result of parameters, show the suitable efficiency of multi-wall carbon nanotube in adsorption Oxymetholone. However, multi-wall carbon nanotube, has the ability to intake the steroid drugs in alive creatures, and or, with steroids adsorption by multi-wall carbon nanotube, and direct the drugs to the interested cells.
Reference
- H. L. J, Makin,Steroid Analysis,D.B.Gwer Editor.
- McMurry, John. Organic chemistry, 5th, ed,( 2000): 366.
- Product Information: Anadrol-50 Oxymetholone. Alaven Pharmaceuticals, Marietta, GA, 2006.
- John H. Lehman,Mauricio Terrones.Elisabeth Manfield.Katherine E. Hurst, Vincent Meunier.carboon, (2011) :492581-2602
- W. A. De Heer, W. S. Bacsa, A. Chatelain, T. Gerfin, R. Humphrey Baker, Nanocapillarity and chemistry in carbon nanotubes, Science(1995) 268: 845.
- M. Meyyappan, Carbon Nanotubes Science & Applications, CRC Press, .2005
- R. H. Baughman, A. A. Zakhidov, W. A. De Heer, Carbon nanotubes- The route toward application, Science.(2002) 297: 787.
- H.Yucel ;N. kincal. direction metod on the adsorption characterisc of activated carbon from kyaya senegalensis fruts. (2009).
- F. Abdulrahman ; L. Hassan ;U. Itodo and S.Maigandi. Nigeria J Basic and Applied Sci .(2008).16(2): 256 – 261.
- A.Zahangir; A. Suleyman ;K. Noraini .,production of activated carbon from oil palm empty fruit Bunch for Zn removal, Conference proceedings 12th Int Water Tech conf. IWTC12 Egypt. (2008) :373-383
- J.Monika ;V. Garg; K. Kardirvelu ; J .Hazardous Materials (2009) 162:365 – 372.
- BH. Hameed ;AM. Din; AL.Ahmad. J Hazardous Materials (2006) 137(3):695-9
- D.Das ; M. Mohanty ; N.,Biswas “Adsorption of phenol from aqueous solutions using activated carbons prepared from Tectona grandis sawdust by ZnCl2 activation”, Chemical Engineering. J .(2005) 115: 121–131

This work is licensed under a Creative Commons Attribution 4.0 International License.









