Interpenetrating Network Polymer Hydrogels of Sodium Alginate and Poly(acrylic acid) for Controlled Release of Prazosin Hydrochloride
Hossein Hosseinzadeh
Department of Chemistry, Payame Noor University, 19395-4697, Tehran, Iran.
In this study, a novel interpenetrating polymer network (IPN) hydrogel composed of crosslinked sodium alginate and poly(acryl acid) was prepared for transdermal delivery of an anti-hypertensive drug, prazosin hydrochloride. Due to the reversible swelling behavior of the hydrogels, the synthesized networks can sense the environmental pH change and achieve an oscillatory release pattern. Using drug prazosin hydrochloride as a model molecule, the in vitro controlled drug-release behaviors of these hydrogels were investigated.
KEYWORDS:sodium alginate; acrylic acid; drug delivery; interpenetrating network polymer
Download this article as:| Copy the following to cite this article: Hosseinzadeh H. Interpenetrating Network Polymer Hydrogels of Sodium Alginate and Poly(acrylic acid) for Controlled Release of Prazosin Hydrochloride. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Hosseinzadeh H. Interpenetrating Network Polymer Hydrogels of Sodium Alginate and Poly(acrylic acid) for Controlled Release of Prazosin Hydrochloride. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23944 |
Introduction
Drug release from solid matrices systems, made of polymer(s) and drug(s), is a basic concept for studies on controlled drug delivery. The most interesting class of polymers in this application is given by hydrogels, also pH-sensitive ones. Hydrogels are special soft and pliable polymeric materials that can be absorb large quantities of water, saline or physiological solutions while the absorbed solutions are not removable even under pressure [1].
Hydrogels are formed from hydrophilic synthetic polymers and many natural polymers such as proteins and polysaccharides. Natural polymer gels are useful for pharmaceutical fields such as controlled delivery devices because of their non-toxic, low cost, free availability, biocompatibility and biodegradability.
Recently, drug delivery systems based on natural hydrogels have been extensively explored to achieve the higher concentration of drugs in the specific region or tissue and the controlled release profile for extended time periods [2-5].
Interpenetrating polymer network (IPN) hydrogels are an intimate combination of two polymers both in the same network, which is obtained when at least one polymer is synthesized and/or crosslinked independently in the immediate vicinity of the other [6].
Prazosin hydrochloride (Figure 1) is a widely used drug for the treatment of hypertension and congestive cardiac failure. The drug is extensively metabolized (>99%) by first pass metabolism. The bioavailability after oral administration is 57% with a shorter biological half-life of 2.9 h. Therefore, it requires multiple dosing to maintain therapeutic drug blood levels. These factors make prazosin hydrochloride, a suitable candidate for transdermal delivery, which avoids first pass metabolism, improves the bioavailability of drug, reduces the frequency of dosing and if toxic symptoms appear, the drug can be withdrawn immediately by removal of membranes. This is especially important for prazosin since there have been reports of sudden collapse after the initial dose.
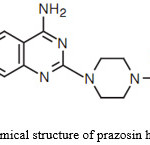 |
Figure 1: Chemical structure of prazosin hydrochloride. |
In the current study we investigated the synthesis and utility of a novel type of IPN hydrogels of sodium alginate with poly(acrylic acid) for the controlled release of prazosin hydrochloride.
Experimental
Preparation of IPN hydrogels
The IPN hydrogels were prepared using sodium alginate (SA) and poly(acrylic acid (PAA). The SA along with PAA was dissolved in double distilled water (total polymer concentration 5%, w/v) at 80 oC and stirred for 1 h, prazosin hydrochloride were added to the polymeric solution and stirred well to get homogeneous solution using magnetic stirrer. Then, a mixture of different quantities of formaldehyde and 1mL of 5N HCl was added slowly and stirring was continued for 2h. The mechanism of formation of IPN structure is schematically shown in Figure 2.
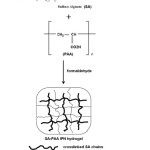 |
Figure 2: Schematic representation of the formation of IPN structure. |
Determination of drug loading
Hydrogel (0.10 g) was immersed in 10 mL of the phosphate buffer solution (pH 7.4) in a 50 mL beaker for completely swelling. The swollen hydrogels were crushed in an agate mortar with a pestle and transferred into a conical flask, and then about 20 mL of the fresh phosphate buffer solution was added to the conical flask and the homogeneous mixture was sonicated for 20 min. The prazosin hydrochloride solution was separated from the mixture after being centrifuged for 20 min at 5000 rpm. The amount of prazosin hydrochloride was determined using UV spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan). The drug loading (%) was calculated using the following equation:
![]()
In vitro drug release
The samples (0.1±0.0001 g) were immersed into 50 mL of the release medium (simulated gastric and intestinal fluids, SGF and SIF) with different pH values (pH 1.2 or 7.4) at 37oC with agitation. At given time intervals, 1 mL of the release medium was removed using a syringe attached with a 0.45 Millipore filter and after suitable dilution the concentration of released drug was measured by UV spectrophotometer at 236 nm. The drug release percent was calculated twice using the following equation:
![]()
where L and Rt represent the initial amount of drug loaded and the final amount of drug released at time t.
Results and Discussion
Drug loading efficiency
In this series of experiments, the drug loading of the hydrogels with different crosslinker content (formaldehyde) were shown in Figure 3. As can be seen, the amount of drug loaded in the hydrogel beads decrease with increasing the content of crosslinker. The greater the crosslinking density, the worse the elasticity of the polymer chains, which could restrict the penetration of prazosin hydrochloride into hydrogel, and then leads to the decrease of the loading amount for drug.
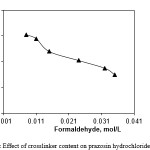 |
Figure 3: Effect of crosslinker content on prazosin hydrochloride loading |
In vitro release behaviour of hydrogels
To determine the potential application of starch-based superabsorbent containing a pharmaceutically active compound, we have investigated the drug release behaviour form this system under physiological conditions. The percent of released drug from the polymeric carriers as a function of pH is shown in Figure 4. The concentration of prazosin hydrochloride released at selected time intervals was determined by UV spectrophotometer. The amount of prazosin hydrochloride released in a specified time from the starch-based hydrogels decreased at pHs lower and higher than pH 8. At acidic pH values, electrostatic repulsion between the carboxylic acid groups of backbone is low, thus decreases gel swelling and minimizes release of cephalexin via diffusion. However, in alkaline media the presence of OH– increases the electrostatic repulsion between carboxylate groups, thus increases the gels swelling degree and so the release of cephalexin increased.
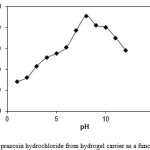 |
Figure 4: Release of prazosin hydrochloride from hydrogel carrier as a function of pH at 37 oC. |
The release rate experiments were also performed in SFG (pH 1.2) and SIF (pH 7.4) solutions at 37 oC (Figure 5). As can be seen from Figure 5, when pH of the medium is 1.2, the cumulative release ratio of prazosin hydrochloride from the test hydrogels is below 35% at the end of the experiment (24 h), whereas almost 82% of the loaded drug is released within 15 h in pH 7.4 medium. Again, these results indicate that the higher swelling ratios of the hydrogel create larger surface areas to diffuse the drug. In basic solutions (pH 7.4), the electrostatic repulsion between COO– anions of grafted poly (sodium acrylate) on the hydrogel accelerates the release of prazosin hydrochloride from the hydrogel.
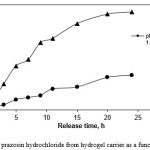 |
Figure 5: Release of prazosin hydrochloride from hydrogel carrier as a function of time and pH. |
References
- K. Park, W.S.W. Shalaby, H. Park, Biodegradable Hydrogels for Drug Delivery, Technomic, New York, 1993.
- V. Raghavendra, V. Kulkarni, S. Biswanath, Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. International Journal of Biological Macromolecules, 47 (2010) 520–527.
- H.Y. Zhou, Y.P. Zhang, W.F. Zhang, X.G. Chen, Biocompatibility and characteristics of injectable chitosan-based thermosensitive hydrogel for drug delivery, Carbohydrate Polymers, 83 (2011) 1643–1651.
- S. Hua, H. Yang, W. Wang, A. Wang, Controlled release of ofloxacin from chitosan-montmorillonite hydrogel, Applied Clay Science, 50 (2010) 112-117.
- K. Hori, C. Sotozono, J. Hamuro, K. Yamasaki, Y. Kimura, M. Ozeki, Y. Tabata, S. Kinoshita, Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing, Journal of Controlled Release, 118 (2007) 169-176.
- L.H. Sperling, Interpenetrating Polymer Networks and Related Materials. Plenum Press, New York, 1981.

This work is licensed under a Creative Commons Attribution 4.0 International License.









