Infrared Measurements of Sodium-Lead-Barium-Aluminium-Phosphate Glass
P. Kothari* and P. Durgapal
Department of Chemistry Government P.G. College Gopeshwar, Chamoli - 246 401 (India).
Infrared spectra were measured for the structural study of the Sodium-lead-barium-aluminum phosphate glass since infrared spectroscopy may probe the nature of the bond between the metal cation and its surrounding oxygens, and can distinguish those metal cations which exhibit a predominantly covalent interaction with oxygen from those metal cation which exhibit mainly ionic interactions with the polyphosphate anionic chains.
KEYWORDS:Sodium-Lead-Barium-Aluminium Phosphate glass; Infrared spectrum; Alkali and alkaline earth metal oxides
Download this article as:| Copy the following to cite this article: Kothari P, Durgapal P. Infrared Measurements of Sodium-Lead-Barium-Aluminium-Phosphate Glass. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Kothari P, Durgapal P. Infrared Measurements of Sodium-Lead-Barium-Aluminium-Phosphate Glass. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24078 |
Introduction
Phosphate Glasses are both scientifically and technologically important materials because they generally offer some unique physical properties better than other Glasses1. Phosphate glass exhibit very important physical properties such as low melting temperature, high thermal expansion coefficient, low glass transition temperature, low softening temperature and high UV transmission2,3. Despite their solubility, the low processing temperature has led these glasses to be used in applications such as glass to metal seals, low temperature enamels for metals and for optical elements4. Also the optical properties like transparency at the excitation and the lasing wavelengths, the spectroscopic properties of the lasing ion, thermo-optic properties, nonlinear optical properties, the fundamental absorption edge and absorption by multiphonon processes depend upon the composition of inorganic glass forming systems and modifiers5,6-11. The choice of suitable glass formers and modifiers help in tailoring the laser glass to meet the specific requirements. The basic building blocks of Phosphate glasses are P2O5 tetrahedras. This oxide with strong bonds is called network former. Introducing into the glass matrix so called network intermediates, PbO and Al2O3, enhances Glass strength. Though network intermediates cannot form glass matrix by themselves, they contribute to the glass strength. Finally, glass can be tailored to have desired properties by adding network modifier. In this case the network modifiers are Na2O and BaO. When two modifiers are used, one having higher percentage is known as primary modifier and the other one as secondary modifier. Addition of secondary modifier shifts the IR cut of edge12 toward longer wavelengths making the rare-earth doped glasses highly suitable for fiber amplifier to be used in telecommunication. This paper describes the Infrared spectra of the samples of the glass.
Experimental
A sodium-lead-barium-aluminium phosphate glass was prepared by melt quenching technique. The final composition (by weight) was approximately Na(PO3)6 70% -BaO 15%-PbO 10%-Al2O3 5%. The starting materials were weighed in an analytical electronic balance. This batch material of 10 gm was ground in an agate pestle mortar for 1 hour to attain homogeneity. The ground batch materials were thermally treated for 5 hours in an alumina crucible up to 940 ± 300C. Stirring the melt with an alumina rod from time to time ensured homogeneity of the melt. The melt was quenched by pouring it into a rectangular shaped brass mould placed on a preheated (1900C) heavy Copper plate. After 12 hours the Glass specimens were taken out.
The Glass specimens so prepared were annealed for 2 hours at 2500C, so as to remove stresses and to give them thermal stability and strength. They were than polished on all sides by mechanically driven device containing a flat covered with polishing cloth. For initial and final polishing, CeO2 and ZrO2 were used as polishing medium respectively. Water was used as coolant for polishing. The polished specimens were again annealed in the muffle furnace at 2500C for 2 hours to remove mechanical stresses, which might be developed during polishing. The Phosphate Glass specimens so prepared were of good optical quality and transparent.
Infrared spectra were taken on a Fourier transform-infrared spectrometer (Perkin Elmer- Spectrum RX-I) using the KBr pellet method. Glass pellets for the infrared investigation were prepared by mixing thoroughly ~ 4 mg glass powder with ~ 200 mg dried KBr powder and compressing the resulting mixture in an evacuable die under 10 ton pressure for 5 min in order to yield transparent discs suitable for mounting in the spectrometer. The infrared spectrum were recorded in the range 400-4000 cm-1.
Results and Discussion
The absorption bands in the IR spectrum shown in Fig. 1 may be made by comparison of our data with past literature13,14. The absorption band at 3432 cm -1 and 1653 cm-1, showing that only a small amount of water is present in the glass, can be attributed to the stretching and bending vibration of either free OH groups or free H2O molecules. The water has no substantial effect on the structure of the glass15. The characteristic features of SLBAP glass spectrum are the stretching of the doubly bonded oxygen vibration n (P=O) modes at 1270 cm-1 16,17, the PO2 symmetric stretching vibration bond (nS PO2) at 1092 cm-1 18, the nas of P-O-P groups at 886 cm-1 and the bending vibration (d) of P-O bonds at 522 cm-1 19.
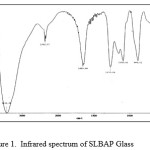 |
Figure 1: Infrared spectrum of SLBAP Glass Click here to View figure |
The structure of a phosphate glass is generally described by the Q(n) groups which are basic structure units (PO4 tetrahedra), where (n) is the number of bridging oxygen atoms per PO4 tetrahedron. It is shown from the above infrared study of the sodium-lead-barium-aluminum phosphate glass that the active species in the glass are PO43- ions. The glass can be viewed as consisting of polymeric chains of PO4 tetrahedra bonded to adjacent tetrahedra via bridging oxygens. These polyphosphate chains are linked together by bonding between the metal cations and non-bridging oxygens of the tetrahedra.
Conclusions
The infrared spectrum of the glass indicates that the active species in the glass are PO43- ions, and that there is characteristic P=O absorption band around 1270 cm -1 in the infrared spectrum, indicating that the oxygen bonding in the glass is mainly P-O-P. Therefore, the Sodium-lead-barium-aluminium phosphate glass is predominantly made up of long chain polymeric phosphate anions which are connected to one another by P-O-P. Infrared spectra shows that due to the addition of BaO as network modifier, a shift in position of the band n (P=O) and ns PO2 occurs and decreases the formation of the end groups in phosphate chain structure.
References
- Tongwei L, Zhengxin T, Weiwei J and Xiaoyang G, Engineering Materials, 368, 1446-1448, (2008).
- Franks K, Abrahams I, Georgiou G and Knowles J C, Biomaterials, 22, 497, (2001).
- Talib Z A, Loh Y N, Sidek H A A, Yusoff M D W, Yunus W M M and Shaari A H, Ceramic International, 30, 1715, (2004).
- Shih P Y, Yung S W and Chin T S, J. Non Cryst. Solids, 224, 143, (1998).
- Weber M J, Ziegler D C and Angell C A, J. Appl. Phys., 53, 4344, (1982).
- Weber M J, J. Non Cryst. Solids, 47, 117, (1982).
- Neuroth N, Soc. Photo-Opt. Instr. Engin., Bellingham, WA, 66, 400, (1983).
- Tick P A, Phys. Chem. Glasses, 23, 54, (1982).
- Rapp F C, Handbook of Laser Science and Technology, M J Weber, CRC Press, Boca Raton, Vol. V, (1988).
- Lucas J, Chathanashuk M, Poulain M, Brun P and Weber M J, J. Non Cryst. Solids, 27, 273, (1978).
- Auzel A, Michel J C, Flahaut J, Loireau-Lozac’h A M and Guittard M, Comp. Rend. Series C, 21, 291, (1980).
- Corbridge D E C and Lowe E J, J. Chem. Phys., 493, (1954).
- Higazy A A and Bridge B, J. Mater. Sci., 20, 2345, (1985).
- Gervais F, Blin A, Massiot D, Coutures J P, Chopinet H M and Naudin F, J. Non-Cryst. Solids, 89, 384, (1987).
- Scholze H, Glass Ind., 47,11, 622, (1966).
- Moustapha Y M, El-Egili K, J. Non-Cryst. Solids, 240, 144–153, (1998).
- Chowdari B V R, Tan K I, Chia W T, Gopalakrishnam R, J. Non-Cryst. Solids, 119, 95, (1990).
- Byun J O, Kim B H, Hong H, Jung H J, Lee S W and Izyneev A A, J. Non-Cryst. Solids, 190, 288-295, (1995).
- Shih P Y, Yung S W, Chin T S, J. Non-Cryst. Solids, 244, 211-222, (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









