Influence of Soluble Salt Matrix on Mechanochemical Synthesis of Lanthanum Cobaltate Nanoparticles
Supachai Sompech¹ and Apinon Nuntiya²*
1Department of Physics and Materials Science, Faculty of Science, Chiang Mai University, Chiang Mai (Thailand).
2Department of Industrial Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai (Thailand).
Mechanochemical reaction of lanthanum cobaltate (LaCoO3) nanoparticles was performed by milling mixtures of LaCl3, CoCl2 and Na2CO3 with no addition and addition of soluble salt matrix (NaCl). The purposes of this work were to synthesize LaCoO3 nanoparticles via mechanochemical process and to study the effect of soluble salt matrix on the average particle size and specific surface area. The three-stage process consisting of mechanical milling, subsequent heating and washing with distilled water was used to synthesize of LaCoO3 nanoparticles. The results indicated that the average particle size (D[4,3]) and the specific surface area are as a function of the soluble salt matrix concentration. Moreover, SEM images revealed that the addition of soluble salt matrix can improve the dispersion of LaCoO3 nanoparticles.
KEYWORDS:Mechanochemical; Lanthanum cobaltate; Soluble salt matrix; Nanoparticles
Download this article as:| Copy the following to cite this article: Sompech S, Nuntiya A. Influence of Soluble Salt Matrix on Mechanochemical Synthesis of Lanthanum Cobaltate Nanoparticles. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Sompech S, Nuntiya A. Influence of Soluble Salt Matrix on Mechanochemical Synthesis of Lanthanum Cobaltate Nanoparticles. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23878 |
Introduction
LaCoO3 has many practical applications owing to its excellent physical and chemical properties. The LaCoO3-based materials have been known to show high catalytic properties for oxidation of carbon monoxide, methane, hexane, and toluene1-4. Thus, it can be used as the catalyst for combustion, automobile exhaust and waste gas purification5-7. Moreover, it could be used as electrode materials for solid-electrode fuel cells and gas sensors8,9. The syntheses of LaCoO3 have been achieved by many methods including conventional ceramic powder technology which leads to low the specific surface area, poorly active materials, requires high temperatures and long calcination periods. To overcome these limitations, several techniques were developed including sol–gel4,6, amorphous heteronuclear complex decomposition synthesis10, the polymerizable complex method11, combustion synthesis12, the molten chloride flux method13, the alkaline coprecipitation method14 and electrochemical oxidation15. These methods can provide products of fine and homogeneous particles with high the specific surface area, but the processes are generally complicated and the reagents used are very expensive.
Recently, mechanochemical processing has been used for the synthesis of ultrafine powders. In this process chemical precursors undergo reaction, either during milling or during subsequent low temperature heat treatment, to form a composite powder consisting of fully dispersed ultrafine particles embedded within a soluble salt by-product. The ultrafine powder is then recovered by removing the salt by-product with a simple washing procedure. This technique has successfully been used to synthesize a wide variety of materials including transition metals16, magnetic intermetallics17, sulfide semiconductors18, and oxide ceramics19-21. The mechanochemical processing technique allows significant control to be exercised over the characteristics of the final washed powder. For example, the average particle size can readily be controlled through the use of inert diluents and post-milling heat treatments. Furthermore, the simultaneous formation of ultrafine particles with an intervening salt matrix suggests that agglomerate formation can more readily be avoided than is possible with other synthesis techniques since the salt matrix inherently separates the particles from each other during processing. In addition, Achimovičová et al.22 have been reported that the effect of soluble salt matrix on the nanoparticles of lead sulphide (PbS) prepared by mechanochemical processing. In this study revealed that the addition of soluble salt matrix can be improved the specific surface area, the average particle size and homogeneity of PbS particles were synthesized.
The purpose of this research was to study of the effect of soluble salt matrix addition on the average particle size and the specific surface area of LaCoO3 nanoparticles which synthesized by mechanochemical method.
Experimental
Materials and synthesis
Starting materials used in the experiment were LaCl3 (Fluka, 99.0%), CoCl2 (Sigma-Aldrich, 97%), Na2CO3 (Fluka, 99.0%) and NaCl (Merck, 99.9%). All the starting materials were dried at 80oC for 2 h. The NaCl was used as soluble salt matrix and added to the starting powders at different concentrations. The mixture of starting powders was milled by planetary ball mill (Retsch, PM 100) at speed 300 rpm under atmospheric. The mechanochemical processing was used to synthesis of LaCoO3 nanoparticles under condition with no addition of soluble salt matrix and performed according to the equation (1). On the other hand, the addition of soluble salt matrix was performed according to equations (2) and (3). The weight and the molar ratio between the reactants were selected empirically in the initial powder mixture.
Twenty grams mixture was put in a stainless steel pot of 250 cm3 inner volume with fourteen stainless steel balls of 20 mm diameter and milled for 2 h. The ground sample was calcined at 600oC with a heating rate of 10oC/min for 90 min, followed by washing with distilled water to remove NaCl phase from by-product. Then, the sample was dried in an oven at 110oC for 2 h.
LaCl3 + CoCl2 + 2.5Na2CO3 + 0.25O2 LaCoO3 + 5NaCl + 2.5CO2 (1)
LaCl3 + CoCl2 + 2.5Na2CO3 + 0.25O2 + 2NaCl LaCoO3 + 7NaCl + 2.5CO2 (2)
LaCl3 + CoCl2 + 2.5Na2CO3 + 0.25O2 + 4NaCl LaCoO3 + 9NaCl + 2.5CO2 (3)
Characterizations
The milled, calcined and washed samples were characterized by X-ray diffraction analysis (Philips X’Pert), using Cu-Ka radiation in an angular range from 2q = 5o to 80o, to identify the phases existing in the product. The average particle size was determined using a laser diffraction method fitted with a wet sampling system (Mastersizer S, Malvern), this particle diameter reported was calculated using volume distribution (D[4,3]). The specific surface area (SSA) of the sample was measured by a nitrogen gas adsorption instrument (Quantachrome, Version 1.11) based on the BET method. Morphology and microstructure of the prepared sample was observed by scanning electron microscope (SEM) and analyzed the chemical composition by energy dispersive spectrometer (EDS) technique (JSM-6301F, JEOL).
Results and Discussion
Fig. 1 shows XRD patterns of samples before and after milled at speed 300 rpm for 2 h by grinding mixture of LaCl3, CoCl2 and Na2CO3. Fig. 1(a) shows peaks which associated with LaCl3, CoCl2 and Na2CO3 mixture before milling and their peaks intensity decrease gradually and vanished after milled for 2 h. However, Fig. 1(b) shows XRD pattern of sample with no addition of soluble salt matrix accorded to the reaction equation (1). It clearly shows the new peaks of NaCl and these peaks correspond to the standard powder diffraction patterns (JCPDS) of NaCl file No. 05-0628. The appearance of the NaCl phase indicated that displacement reactions between LaCl3, CoCl2 and Na2CO3 occurred during milling and other phases such as various types of carbonate and possibly unreacted starting compounds, are present in the form of amorphous or minor phases in the ground product23. This reaction occurred indicates that the mechanochemical (MC) solid-state reaction can be proceeded by grinding mixture of starting material powders using a high-energy ball mill at room temperature19,24-26. In addition, the NaCl phase was formed in the ground product and it plays a big role to disperse or enable well separate nanoparticles of LaCoO327. Moreover, XRD pattern of sample with addition of soluble salt matrix at the different concentrations which accorded to the reactions equations (2) and (3) also shows high intensity of NaCl diffraction peaks as shown in Figs. 1(c) and 1(d). Increasing of the intensity peaks were increased the concentration of soluble salt matrix addition, this resulted due to increase in the crystalline volume fraction of NaCl which added into the initial starting mixture during the grinding process28.
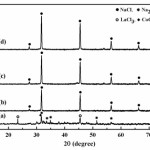 |
Figure 1: XRD pattern of reactant mixture and milling samples; (a) starting mixture before milling, (b) no addition of soluble salt and milled for 2 h, (c) addition of 2 mol soluble salt and milled for 2 h and (d) addition of 4 mol soluble salt and milled for 2 h |
Fig. 2 shows XRD pattern of samples after milled and calcined at 600oC for 90 min. The XRD pattern of all samples clearly revealed the new peaks of LaCoO3 which were observed after heat treatment and associated with the NaCl phase. These results indicate that the reaction between the starting materials occurred during calcinations which can be given by equations (1)-(3). The presence of LaCoO3 phase after heat treatment as shown in Figs. 2(a), 2(b) and 2(c), indicates that the milling of starting powders by mechanochemical processing leads to form of desired phase at low temperature (600oC)27-30.
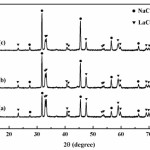 |
Figure 2: XRD pattern of samples after milled for 2 h and heated at 600oC; (a) no addition of soluble salt matrix, (b) addition of 2 mol NaCl and (c) addition of 4 mol NaCl |
Fig. 3 shows XRD pattern of samples after washed with distilled water. It was found that the peaks of LaCoO3 are present in all conditions and do not have any impurities. Therefore, it can be concluded that the NaCl phase in the by-product was removed out by the washing operation27,28. Moreover, the intensity of LaCoO3 peaks show slightly broadening peak and decrease gradually with an increasing in concentrations of soluble salt matrix addition as shown in Figs. 3(a), 3(b) and 3(c). Decreasing of the intensity attributed that the NaCl phase within the starting powders can be prevent reaction occurring, due to the soluble salt matrix separate the reactants, reducing the reaction volume between the reactant particles, leading to a decrease in reaction rate and the rate of heat generation in the calcination process25,28. Furthermore, the XRD patterns of LaCoO3 peaks correspond to the standard powder diffraction patterns (JCPDS) file No. 25-1060 with perovskite-type structure.
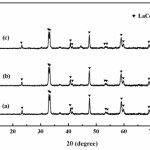 |
Figure 3: XRD pattern of samples after washed with distilled water; (a) no addition of soluble salt matrix, (b) addition of 2 mol NaCl and (c) addition of 4 mol NaCl |
Table 1 shows the effect of soluble salt matrix addition on the average particle size (D[4,3]) and the specific surface area of samples. The results show that the average particle size and specific surface area of samples depend on the concentration of soluble salt matrix which added in the initial reactants mixture. The average particle size was decreased with increased in the concentration of NaCl, while specific surface area was increased. The results are accordance with previously studies of Dodd and McCormick28 and Hector et al.31 which found that the addition of soluble salt matrix to the reactant mixtures can be reduced the average particle size and crystallite size. The average particle size of LaCoO3 with no addition of soluble salt matrix is 3.14 mm, while the samples with addition of 2 mol NaCl and 4 mol NaCl decrease down to 2.94 and 2.63 mm, respectively. On the other hand, the specific surface areas were increased from 25.67 up to 26.93 and 27.48 m2/g when addition of 2 mol NaCl and 4 mol NaCl, respectively. Decreasing of the average particle size and increasing the specific surface area due to the effect of soluble salt matrix in the initial reactant mixture. The salt matrix is not only separate the LaCoO3 particles to prevent agglomeration, but also enable well dispersed of LaCoO3 particles after calcinations24,25,28.
Table 1: Average particle size and specific surface area of samples
| Samples | Average particle size (D[4, 3]) Specific surface area
(mm) (m2/g) |
| LaCoO3 3.14 ± 0.02 25.67 ± 0.01
LaCoO3 + 2NaCl 2.94 ± 0.02 26.93 ± 0.01 LaCoO3 + 4NaCl 2.63 ± 0.02 27.48 ± 0.01 |
|
The morphology of samples after washing was investigated by scanning electron microscope (SEM). SEM images clearly illustrated that the significant influence of the soluble salt matrix addition can improve the dispersion of LaCoO3 nanoparticles. These results indicated that the existence of soluble salt matrix phase in the initial reactant mixture will prevent the agglomeration of particles or separate the LaCoO3 nanoparticles during calcination. Fig. 4(a) shows SEM micrograph of the LaCoO3 nanoparticles which no addition of soluble salt matrix, is comprised of highly agglomerate particles. In the contrary, Figs. 4(b) and 4(c) show SEM micrographs of LaCoO3 nanoparticles which addition of 2 mol NaCl and 4 mol NaCl show loosely agglomerated of particles and clearly separate of particles. Furthermore, EDS spectrums as shown in Fig. 5 illustrated evident peaks of La, Co and O which accord to chemical elements of LaCoO3 nanoparticles.
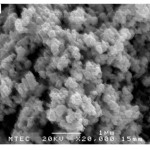 |
Figure 4: SEM micrograph of samples after washing; (a) no addition of soluble salt matrix |
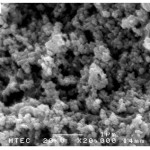 |
Figure 4(b): addition of 2 mol NaCl |
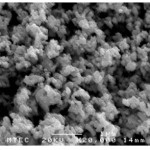 |
Figure 4(c) addition of 4 mol NaCl |
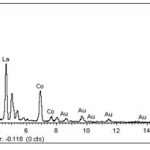 |
Figure 5: EDS spectrums of LaCoO3 after washing |
Conclusions
LaCoO3 nanoparticles can be successfully prepared by heat treatment of the milled powder obtained by mechanochemical reaction of LaCl3, CoCl2, and Na2CO3 with NaCl as a soluble salt matrix. The soluble salt matrix can separate and prevent the agglomeration of LaCoO3 nanoparticles after calcinations at 600oC. The addition of the soluble salt matrix in the reactant mixture can reduce the average particle size and increase the specific surface area. Moreover, SEM micrographs show loosely agglomerate nanoparticles.
Acknowledgments
I would like to thank the Commission on Higher Education, Thailand for supporting by grant fund under the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree for this research. Also, I would like to thank the Faculty of Science and Graduate School of Chiang Mai University for partial financial support.
References
- Salker A. V., Choi N. J., Kwak J. H., Joo B. S. and Lee D.-D., Sens. Actuators, B, 106: 461 (2005).
- Cimino S., Landi G., Lisi L. and Russo G., Catal. Today, 105: 718 (2005).
- Szabo V., Bassir M., Gallot J. E., Van Neste A. and Kaliaguine S., Appl. Catal., B, 42: 265 (2003).
- Alifanti M., Florea M., Somacescu S. and Parvulescu V. I., Appl. Catal., B, 60: 33 (2005).
- Kucharczyk B. and Tylus W., Catal. Today, 90: 121 (2004).
- Kirchnerova J., Alifanti M. and Delmon B., Appl. Catal., A, 231: 65 (2002).
- Zhu Y., Tan R., Feng J., Ji S. and Cao L., Appl. Catal., A, 209: 71 (2001).
- Ohno Y., Nagata S. and Sato H., Solid State Ionics, 3-4: 439 (1981).
- Shimizu Y. and Yamashita N., Sens. Actuators, B, 64: 102 (2000).
- Zhu Y., Tan R., Yi T., Ji S., Ye X. and Cao L., J. Mater. Sci., 35: 5415 (2000).
- Popa M., Frantti J. and Kakihana M., Solid State Ionics, 154-155: 135 (2002).
- Singanahally T. A., Meiyappan M. and Kashinath C. P., J. Mater. Chem., 7: 2499 (1997).
- Armelao L., Bandoli G., Barreca D., Bettinelli M., Bottaro G. and Caneschi A., Surf. Interface Anal., 34: 112 (2002).
- Liang J. J. and Weng H.-S., Ind. Eng. Chem. Res., 32: 2563 (1993).
- Matsumoto Y., Sasaki T. and Hombo J., Inorg. Chem., 31: 738 (1992).
- Ding J., Tsuzuki T., McCormick P. G. and Street R., J. Phys. D, 29: 2365 (1996).
- Liu W. and McCormick P. G., Nanostruct. Mater., 12: 187 (1999).
- Tsuzuki T., Ding J. and McCormick P. G., Physica B, 239: 378 (1997).
- Dodd A. C., Raviprasad K. and McCormick P. G., Scripta Mater., 44: 689 (2001).
- Ding J., Tsuzuki T. and McCormick P. G., J. Am. Ceram. Soc., 79: 2956 (1996).
- Ding J., Tsuzuki T. and McCormick P. G., Nanostruct. Mater., 8: 75 (1997).
- Achimovičová M., Godočíková E., Baláž P., Kováč J. and Šatka A., Rev. Adv. Mater. Sci., 18: 216 (2008).
- Muroi M., Street R. and McCormick P. G., J. Appl. Phys., 87: 3424 (2000).
- Cukrov L. M., Tsuzuki T. and McCormick P. G., Scripta Mater., 44: 1787 (2001).
- Tsuzuki T. and McCormick P. G., Appl. Phys. A, 65: 607 (1997).
- Tsuzuki T. and McCormick P. G., Acta Mater., 48: 2795 (2000).
- Ito T., Zhang Q. and Saito F., Powder Technol., 143-144: 170 (2004).
- Dodd A. C. and McCormick P. G., Acta Mater., 49: 4215 (2001).
- Tsuzuki T. and McCormick P. G., Scripta Mater., 44: 1731 (2001).
- Dodd A. C. and McCormick P. G., Scripta Mater., 44: 1725 (2001).
- Hector A. L., Henshaw G., Komarov A. V. and Parkin I. P., J. Mater. Process. Technol., 77: 103 (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









