Improvement of Physico-Chemical Properties of Silk Fibre Modified with Acid Anhydride Monomer under Redox Initiation System
Md. Ibrahim H. Mondal* and Md. Mashiur Rahman Khan
Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi 6205, Bangladesh.
The physico-chemical properties of silk fibre can be improved by using acid anhydride monomers, e.g. phthalic anhydride and succinic anhydride in presence of potassium peroxodisulfate (K2S2O8) as an initiator. The anhydride monomer was added to silk fibre by graft-copolymerization that was followed by using the parameters, i.e. monomer concentration, initiator concentration, modification time and modification temperature. The graft yield increased with the increase of monomer concentration from 20 to 90% and initiator concentration from 0.2 to 1.8% and then it decreased. Time and temperature also had great influenced on the rate of reaction and maximum graft yield was obtained upto 90 oC within 2.5 h. The graft yield at optimized condition was 16.6% for phthalic anhydride and 14% succinic anhydride. The effect of various external influences such as temperature and sunlight on degummed and modified silk fibres was also observed. The loss in tenacity of degummed silk is comparatively higher than that of modified silk fibre. The dyeing characteristics as well as fastness properties of modified silk is better than that of degummed one.
KEYWORDS:Silk fibre; acid anhydride monomers; grafting; tenacity; colour fastness
Download this article as:| Copy the following to cite this article: Mondal M. I. H, Khan M. M. R. Improvement of Physico-Chemical Properties of Silk Fibre Modified with Acid Anhydride Monomer under Redox Initiation System. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Mondal M. I. H, Khan M. M. R. Improvement of Physico-Chemical Properties of Silk Fibre Modified with Acid Anhydride Monomer under Redox Initiation System. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23875 |
Introduction
Silk is termed as “Queen of Fabrics” for its unique characteristics and have never been equaled in any other fibre1. Silk fabrics are luxurious in appearance and lead themselves readily to style changes2. In olden times, silk was reserved for persons of high position and only such people were allowed to wear it. Even today, use of silk materials is regarded as a demonstration of the artistic taste of wealthy people 3.
But silk possesses some inferior textile performances, such as photo-yellowing, wash and wear abrasion resistance, crease recovery, rub resistance, colour fastness, oil and water repellency, etc which seriously limit its general use4,5. The characteristics of silk can be improved by chemical modification technique. Among the various methods available for the modification of silk, graft-copohymerization onto silk fibre with acid anhydride monomers appear and are recently used in industrial applications5.
A large number of acid anhydride monomers have been applied onto silk and they influence on the fibre properties. Among the acid anhydride monomers, phthalic anhydride and succinic anhydride are the most popular and useful grafting agents. So, they have been played a significant role to minimize the undesirableness and to improve the above inferior textile performances of silk fibre6.
Effect of sunlight on the tenacity of silk fibre
The effect of sunlight on the tenacity of degummed and modified, i.e. phthalic anhydride and succinic anhydride modified silk fibre is shown in Fig. 6. From the Fig. 6 it is evident, the tenacity of both degummed and modified silk fibre decreased with the progress of exposure time under sunlight in open air. Silk is a polymer of amino acids having polypeptide linkage. The glycidal radicals formed in silk fibroin by the irriadiation immediately react with atmospheric oxygen and changes to peroxy radicals, i.e. photo oxidative degradation occur17. After 300 h exposure, the loss in tenacity of degummed silk is 52.4% which is higher than that of modified silk, e.g. 34.2% for phthalic anhydride and 39.4% for succinic anhydride at the same time of exposure. This happens, probably, modification with acid anhydride monomers, the reactive groups present in silk fibre are blocked by chemical bonds with anhydride monomers. As a result photo-oxidative degradation occurs slightly and the fibre is protected from the harmful effect of sunlight. It is also observed, the loss in tenacity of each modified fibre on exposure to sunlight in air is not same. The plausible explanation of such behaviour is that the physico-chemical properties of grafted silk fibre largely depends on the chemical characteristics of the functional side groups (reactive sites) carried by the acid anhydride monomers. Higher percentage of grafting gives comparatively lower loss tenacity. However, the loss in tenacity of degummed silk is higher than that of modified silk fibre.
In the present work, attempts have been taken to modify the silk fibres with acid anhydride monomers, i.e. phthalic anhydride and succinic anhydride initiated under k2S2O8 redox system. In the fact of economic view, the optimum modification conditions such as monomer concentration, initiator concentration, modification time and temperature were find out. Graft yield was determined on the basis of weight gain of the treated fibre. The effects of temperature and sunlight on the tenacity and colour fastness characteristics of the fibres have been also investigated.
Experimental
Raw silk fibre of improved multi-voltine variety was collected from Bangladesh Sericulture Research and Training Institute (BSRTI) Rajshahi, Bangladesh. For experiment the fibre was sized from the technological section of BSRTI. Degumming of raw silk was carried out in two bath systems with soap solution of strength 3.5 g/l at pH 10.0-10.5 and 95-100ºC for 1 hr. in the fibre-liquor ratio of 1: 30. Phthalic anhydride (PA, C6H4(CO)2O) and succinic anhydride (SA, C4H4O3) was purified. All other reagents used were of analytical grade.
Grafting Procedure
Grafting of silk fibre was carried out in 100 ml Stoppered Erlenmeyer flask. Polymerization was done with 20-90% monomer and 0.2-1.8% initiator at 40-90ºC for 0.5 – 3.0 h in the fibre-liquor ratio of 1:50. After completion of the reaction the fibre was washed well by extraction with warm water followed by warm methanol to remove the loosely adhering polymer, then washed with water and dried7,8.
The graft yield was calculated from the increased in weight of the original silk fibre after grafting as follows:
Graft yield (%) = (W2 – W1)/ W1 Í 100
Where W1 and W2 denote the weight of the original silk fibre and the grafted silk fibre, respectively.
Measurement of Tenacity
Tenacity measurement of silk fibre was carried out by Torsee’s Schopper type OS-100 Tensile Tester. Breaking load was measured according to the previous work9,10. Tenacity was calculated by the following formula11 and is expressed as gm/denied.
Tenacity = Average breaking load / Denier
Where, Denier = (Weight of the sample in gm/ Length of the sample in metre) ´ 9000
Sunlight exposure for tenacity
By setting on a flat board, the specimen of silk fibre was exposed directly under the sunlight without any protection from weathering, but was protected from rain, dust etc. At the same time and in the same place, silk sample was exposed under the sun for 7 h each day in the month of May to September.
Heat exposure for tenacity
Silk sample was placed directly in an electric oven in presence of air at 25-200 oC for 3 h and the tenacity was measured.
Results and Discussion
Effect of monomer concentration
The effect of monomer concentration of PA and SA on the graft yield was investigated and is shown in Fig.1. The graft yield increases with the increased of monomer concentration upto 80% and 90% for PA and SA, respectively and then it decreased with further increase of monomer concentration. This can be explained that when silk fibre is immersed into a solution of acid anhydride monomer in presence of potassium peroxodisulphate (K2S2O8) as a redox initiator, the silk is attacked by the redox initiator and the free radical is formed on the backbone of the silk fibrion. Carboxyl (- COOH), amino (-NH) and hydroxyl (-OH) side groups of acidic, basic and hydroxyl acid residues are the main reactive sites of silk fibre. These reactive sites interact with anhydride monomers, thus grafting occurred12. Again, the decrease of graft yield with the further increase of monomer concentration may be due to the cross termination of reactive monomer or radical sites. When the concentration of monomer radical increases, the rate of their combination and disproportionation increases faster than the rate of their combination with silk molecules.
From Fig.1, it is observed that the graft yield of PA and SA monomers onto silk fibres are not the same. Graft yield of PA is higher than that of SA. This may be due to the chemical features and reactivity of them towards silk fibre. Chemical structure of the anhydride monomer shows that both PA and SA have anhydride radicals. Besides, PA and SA have benzoyl and acetyl radicals respectively and these two substituents enhance the rate of reaction mechanism6.
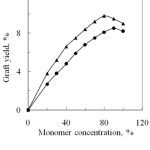 |
Figure 1: Effect of monomer concentration on graft yield of degummed silk fibre.▲, Phthalic anhydride and , Succinic anhydride. |
The acylation of silk fibres with phthalic anhydride resulted in the formation of crosslink’s (mainly hydrogen bonds and/or hydrophobic interactions) between the side groups of the aromatic acid (which come from hydrolyzing PA) and active sites located on adjacent fibroin chains. The carboxyl group freed by phthalic anhydride after reaction with fibroin could only partly contrast the hydrophobicity of the benzoyl group. So, the moisture regain comparatively more than succinic anhydride because it cannot be freed carboxyl group on reaction with fibroin6.
From the Fig. 1, it has been observed that high graft yield was obtained when degummed silk was modified with 80% PA and 90% SA. At the effective concentration the corresponding graft yields are 9.8 and 8.5% respectively. So, the reactivity of PA with silk fibre is higher than SA.
Effect of initiator concentration
The effect of initiator (K2S2O8) concentration on the graft yield of anhydride monomers onto silk fibre is shown in Fig. 2. The graft yield increases with the increased of initiator concentration upto 1.8 and 1.4% for PA and SA, respectively and then it slightly decreased.
Initiator forms an intermediate complex with silk fibre which is dissociated yielding free radicals on the backbone of silk molecules. These free radicals interact with anhydride monomer, thus grafting occurs. With increasing initiator concentration, the number of free radicals increases which enhance graft yield. The decrease of graft yield at higher initiator concentration may be caused by formation of homopolymers over grafting and the termination of growing grafted chain by primary free radicals resulting from the decomposition of the excess of the initiator. The optimum initiator concentration is 1.8% and 1.4% for PA and SA respectively, where the corresponding graft yields are 11.0% and 9.8%.
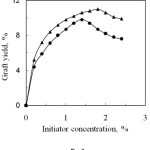 |
Figure 2: Effect of Initiator concentration on graft yield of degummed silk fibre.▲, Phthalic anhydride and , Succinic anhydride. |
Effect of modification time
The effect of treatment time on graft yield is shown in Fig. 3, which evidences that the graft yield increased with the increase of reaction time upto 2.5 h for PA and 1.5 h for SA, beyond which it decreased slightly. The rate of grafting increases with time, at first very sharply, due to a higher grafting reaction and a lower homopolymerization reaction. After the optimum reaction time, the monomer is not assumed to graft with the fibre. The excess monomer is used to form the homopolymer13. Another cause may be due to the partial dissolution of the grafted fibre on prolonged exposure to the high temperature14. The effective modification times for PA and SA are of 2.5 h and 1.5 h respectively, where the corresponding graft yields are of 12.9% and 10.6%, respectively.
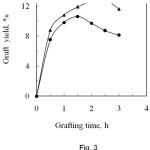 |
Figure 3: Effect of reaction time on graft yield of degummed silk fibre.▲, Phthalic anhydride and , Succinic anhydride. |
Effect of modification temperature
The changing of temperature greatly affects the polymerization reaction and is shown in Fig. 4. From the Fig. 4, it can be observed that the graft yield increased on increasing the temperature upto 90oC both for PA and SA, where the corresponding graft yields are 16.6% and 14.0% respectively. Beyond this temperature, the graft yield slightly decreased. The increase in graft yield may be explained that at higher temperature, the swelling ability of the fibre and the diffusion rate of the monomer from aqueous phase to fibre phase increases resulting in the increase of graft yield15. Therefore, the increase of reaction temperature increases the rate of the polymerization. Again, at higher temperature, the decomposition rate of redox initiator increases resulting in the increase of free radicals on the backbone of silk fibre. As a result more monomer molecule reacts with silk fibre giving high graft yield. At ceiling temperature, the net polymer production is zero, i.e. the propagation and depropagation rates are equal. Above the ceiling temperature, the decrease of graft yield may be explained by polymer degradation phenomenon. In this case the rate of polymer formation is less than that of polymer degradation which gives the low graft yield.
The optimized conditions for grafting of silk fibre with PA and SA are summarized in Table 1.
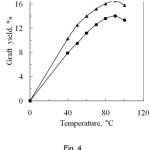 |
Figure 4: Effect of reaction temperature on graft yield of degummed silk fibre.▲, Phthalic anhydride and , Succinic anhydride.
|
Effect of heating on the tenacity of silk fibre
Figure 5 shows the effect of temperature on the tenacity of degummed and modified silk fibres. From the Fig. 5, it can be observed that the tenacity of both degummed and modified silk fibre increased with the rise of temperature upto 100oC for 3 h, then it decreased with further increase of temperature. This may be explained that moisture, present in the amorphous region of silk is removed with the rise of temperature, thus causing increase in the tenacity of silk fibre. The tenacity decreased above 100oC and upto 170oC the decomposition of silk occurs without danger. But silk fibre is rapidly disintegrated at and above 170oC16.This is perhaps at higher temperature, the energy of thermal motion causes fluctuation of the chemical bonds, as a result in the polypeptide chains the bonds become weaker and ultimately, the tenacity of the fibre decreases.
Again, the tenacity of modified silk is higher than that of degummed silk fibre. This is due to grafting of silk fibre with acid anhydride monomers; the crystallinity of silk fibre is reduced due to the incorporation of polypeptide chain and gives an additional strength to silk11. The order of tenacity of PA modified fibre is greater than that of SA modified fibre. It may be explained that higher percentage of graft yield gives the higher strength to silk fibre.
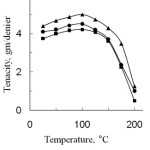 |
Figure 5: Effect of temperature on the tenacity of degummed and grafted silk fibres (Graft yield, PA, 16.1% and SA, 13.6%)■ degummed silk fibre; ▲, Phthalic anhydride and , Succinic anhydride. |
Effect of sunlight on the tenacity of silk fibre
The effect of sunlight on the tenacity of degummed and modified, i.e. phthalic anhydride and succinic anhydride modified silk fibre is shown in Fig. 6. From the Fig. 6 it is evident, the tenacity of both degummed and modified silk fibre decreased with the progress of exposure time under sunlight in open air. Silk is a polymer of amino acids having polypeptide linkage. The glycidal radicals formed in silk fibroin by the irriadiation immediately react with atmospheric oxygen and changes to peroxy radicals, i.e. photo oxidative degradation occur17. After 300 h exposure, the loss in tenacity of degummed silk is 52.4% which is higher than that of modified silk, e.g. 34.2% for phthalic anhydride and 39.4% for succinic anhydride at the same time of exposure. This happens, probably, modification with acid anhydride monomers, the reactive groups present in silk fibre are blocked by chemical bonds with anhydride monomers. As a result photo-oxidative degradation occurs slightly and the fibre is protected from the harmful effect of sunlight. It is also observed, the loss in tenacity of each modified fibre on exposure to sunlight in air is not same. The plausible explanation of such behaviour is that the physico-chemical properties of grafted silk fibre largely depends on the chemical characteristics of the functional side groups (reactive sites) carried by the acid anhydride monomers. Higher percentage of grafting gives comparatively lower loss tenacity. However, the loss in tenacity of degummed silk is higher than that of modified silk fibre.
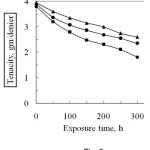 |
Figure 6: Effect of sunlight on the tenacity of degummed and grafted silk fibres (Graft yield, PA, 16.1% and SA, 13.6%).■ degummed silk fibre ▲ Phthalic anhydride and , Succinic anhydride. |
Dyeing Properties
For the dyeing of both degummed and modified silk fibres, two acid dyes, i.e. Acid Red 114 and Acid Orange 52 were used. It was observed that the dye absorption of modified silk fibre is slightly higher than that of unmodified fibre. The plausible explanation of such behaviour is that chemical modification makes the fibre hydrophobic in nature. Due to the hydrophobic nature, the dyability of the fibre increases16. pH of the dye bath plays an important role in dyeing of silk fibre with acid dyes. The optimum pHrange is 3.0 – 3.5 at which maximum dye absorption, highest colour fastness and fibre stability were obtained. Below this pH, the hydrolytic cleavage of peptide bonds occurs18.
Modified and modified dyed silk fibres exhibited better light resistivity than degummed silk fibre when they were exposed to sunlight in open air for 300 hrs. This is due to the atmospheric oxygen cannot react with silk fibre to form peroxy radicals because of blocking the active groups on silk backbone. Again, during washing with soap solution (5 g/l), modified fibre showed better wash fastness at any temperature upto boiling. In acid and alkali spotting, both degummed and modified silk fibres showed the satisfactory results.
Conclusions
Most of the physico-chemical properties of silk fibre are improved by graft copolymerization. Chemical modification gives additional strength to silk fibre to overcome the inferior textile performances. So, it can be widely used in textiles.
References
- Hess, K. P., Textile Fibres and Their Use (6th Edn.), Oxford and IBH Publishing Co., New Delhi, 1978, pp. 222-48, 157-59.
- Mathews, J. M., Application of dyestuffs, John Willey & Sons, Inc., New York, 1920, pp. 1-2, 166, 178, 187.
- Philip, T., Indian Silk- “Why silk is Precious”, 1989, p. 42.
- Tsukada, M., Goto, Y., Freedi, G., Matsumura, M., Shiozaki, H. and Ishikawa, H., J. Appl. Polym. Sci., 44: 2203 (1992).
- Tsukada, M., Freedi, G., Shiozaki, H. and Pusch, H., J. Appl. Polym. Sci., 49: 593 (1993).
- Tsukada, M., Goto, Y., Freedi, G., Shiozaki, H., J. Appl. Polym. Sci., 45: 1189 (1992).
- Rafie, M. H. E., Abdul Hafiz, S. A.,Hasan, S. M. and Hebiesh, A., Polym .Polym. Comp.,2: 100 (1994).
- Hebeish, A., Kantouch, A. and EL-Rafie, M. H., J. Appl. Polym. Sci., 15: 11 (1971).
- Farouqui, F.I.,and Mondal, Md. I. H., The Rajshahi Univ. Studies, 17(B): 1, (1989).
- International Standard ISO 5081-1977(E).
- Haque, M. M. and Habibuddowla, Md., Bangladesh J. Sci. Ind. Res., 15: 64 (1980).
- Tsukada, M.,Melliand English, 8: E287 (1993).
- Mondal, Md. I. H., Farouqui, F. I. and Enamul Kabir, F. M., Cellulose Chem.Technol., 36(5-6): 471 (2002).
- Tripathy, S. S., Jena, S., Misra, S. B., Padhi, N. P. and Singh, B. C., J. Appl. Polym. Sci., 30(4): 1399 (1985).
- Samal, R. K., Nanda, C. N, Satrusallya, S. C. and Nayak, B. L., J. Appl. Polym. Sci., 28:1311 (1983).
- Mathews, J. M.., “Mathews Textile Fibres”(6th Edn.), John Wiley & Sons, Inc., New York, 1954, p. 796.
- Setoyama, K., Nippon Sanshigaku Zasshi, 51(4): 27 (1982).
- Chemical abstract, Vol. 118, 1993, 1420p.

This work is licensed under a Creative Commons Attribution 4.0 International License.









