Immobilization of Chetoceros sp Microalgae with Silica Gel through Encapsulation Technique as Adsorbent of Pb Metal from Solution
Buhani, Suharso and Z. Sembiring
Department of Chemistry, Faculty of Mathematic and Natural Sciences, University of Lampung, Lampung, Indonesia.
Immobilization of Chetoceros sp microalgae with silica as supporting matrix has been carried out through encapsulation technique with using solution of Na2SiO3 sol. Identification of functional groups and surface morphology from adsorbent obtained was performed with utilizing infrared spectrophotometer (IR) and scanning electron microscopy (SEM). Adsorption process of Pb(II) ion on adsorbent was performed with batch method including determination of rate, capacity, and optimum pH of adsorption. Adsorption data found show that adsorption process of Pb(II) ion by Chetoceros sp biomass and Chetoceros sp-silica leans to follow pseudo first order kinetic model with each rate value (k1) of 0.032 and 0.065 min-1. Adsorption capacity (qm) of Pb(II) ion on Chetoceros sp-silica adsorbent is higher than adsorption capacity of Pb(II) ion on Chetoceros sp adsorbent without immobilization with silica.
KEYWORDS:Immobilization; Encapsulation; Chetoceros sp; Adsorption
Download this article as:| Copy the following to cite this article: Buhani B, Suharso S, Sembiring Z. Immobilization of Chetoceros sp Microalgae with Silica Gel through Encapsulation Technique as Adsorbent of Pb Metal from Solution. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Buhani B, Suharso S, Sembiring Z. Immobilization of Chetoceros sp Microalgae with Silica Gel through Encapsulation Technique as Adsorbent of Pb Metal from Solution. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23824 |
Introduction
Contamination of heavy metals in environment is one of serious problems to be controlled currently 1. This case occurs because of increasing of heavy metal use in industry followed by negative effects of spreading waste containing heavy metals in environment 2. As other environmental pollution sources, heavy metals can move as far as possible in the environment and they can be potential problem for our live in the next time. One of heavy metals which is more produced and broadly used is Pb metal. The biggest sources of Pb released to environment are derived from battery manufacturing, acid metal plating and finishing, ammunition, tetraethyl lead manufacturing, ceramic and glass industries printing, painting, and dying3. As other heavy metal characteristics, adsorption of Pb metal in human body is very slow 4, so that accumulation process can be progressive toxicity 5.
In order to handle spreading of Pb metal in the environment, several programs have been applied to reduce spreading and concentration of heavy metals 6 especially Pb metal in the environment as use of algae biomass as effective adsorbent to bind heavy metal ions via adsorption process 7. Algae biomass from several species of algae is effective to bind metal ions from aquatic environment 8-10 because algae biomass contains some functional groups which are play a role as ligand toward metal ions 11,12. But, for the specific adsorbent material, algae biomass has some problems such as; low density and easy to be degraded biologically or chemically. These are to be ineffective to be purposed as a column filling material to be a continually adsorption process 8.
In order to control the weaknesses of algae biomass6 use as adsorbent, immobilization process has been applied with using several supporting matrixes as silica gel 13,14. The algae biomass used is Chetoceros sp microalgae which has big enough abundance in the ocean. In this research, it was performed immobilization of biomass with silica matrix through encapsulation process. Encapsulation technique is very potential because silica matrix can form cage for biomolecules to produce stronger sites for biomolecules 15 and to be also used for immobilization of protein on biomass to keep its spectroscopy characteristics and biological activities 16. Encapsulation process of Chetoceros sp biomass with silica will maintain active groups playing a role as ligands to bind metal ions and to produce chemically stable adsorbent. In order to know success of the encapsulation process, the adsorbent resulted from immobilization process was examined its adsorption characteristics toward Pb(II) ion in solution based on kinetic model and adsorption isotherm.
Experimental
Material and Instrumentation
Chetoceros algae biomass was taken from Lampung Sea Cultivation Bureau (Balai Budidaya Laut Lampung), Indonesia. Na2SiO3, strong acid cation exchange resin, phosphate buffer, Pb(NO3)2, HCl (37 %), and NaOH were ordered from Alba Chemical.
Analytical balance (Mettler AE 160), sieve (with size of 200 mesh), oven (Fisher Scientific), magnetic stirrer, centrifuge (OSK 6474B), pH meter (Orion 4 Star), vacuum pump (Buchi VacR V−500) were used as apparatus in this project. Atomic absorption spectrophotometer (AAS) (Perkin Elmer 3110) was applied to calculate metal concentration. IR spectrophotometer (Prestige Shimadzu) was performed to identify functional groups from adsorbent. SEM-EDX (JSM 6360 LA) was carried out to analyze surface morphology of adsorbent.
Preparation of Chetoceros sp biomass
Cultivation of Chetoceros sp was done for 8 days and it was centrifuged to get biomass. Biomass obtained was placed in solution of 0.12 M HCl, it was agitated for 20 minutes, and it was centrifuged to separate with HCl solution. These procedures were repeated for 2 times furthered with washing by water. Then, it was centrifuged and dried with freeze dryer for 24 hours to get dry biomass which is ready to be used.
Preparation of Chetoceros sp biomass immobilized silica gel
Encapsulation process of algae biomass with silica aquagel: 2.5 mL of Na2SiO3 sol solution were mixed with 5 mL water. The mixtures were added 1.95 mL strong acid of cation exchange resin until solution pH close to 4. Then, resin was separated with filtration. The filtrate obtained was added with HCl 2 M and was stirred by magnetic stirrer until sol was resulted at pH of 2. Then, the sol found was given phosphate buffer with ratio of 1 : 5 (v/v). After mixing, solution was removed into beaker glass to produce gel, added microalgae biomass, and continued with aging the gel for 24 h.
Biosorption process with batch method: to investigate kinetic and thermodynamic aspects, parameters of time effect, concentrations, temperatures, and optimum pH in the biosorption process were evaluated. Therefore, experiments performed consisted of:
Biosorption time: 20 mg of algae biomass which was immobilized were interacted with 25 mL of Pb(II) solution 10 mg L−1 and they were mixed using magnetic stirrer. Interaction time was started from 5-80 minutes. Then, the mixture was centrifuged and filtrate was taken and analyzed metal concentration left in solution by AAS.
Various concentrations: 25 mL Pb(II) solution at various concentrations of 0 – 10 mg L−1was interacted with 20 mg of adsorbent at temperature of 27 oC and stirred at optimum time in experiment of a. Then, it was centrifuged to isolate filtrate and sediment. Concentration of Pb(II) left was measured by AAS.
Interaction of various pH: 25 mL Pb(II) solution with optimum concentration variety was placed into reaction tube containing 20 mg of adsorbent, then it was adjusted pH of 3-8. The mixture was stirred at optimum time (experiment of a). Then, the solution obtained was analyzed by AAS.
Results and Discussion
Adsorbent characteristics
Adsorbent characteristics obtained from Chetoceros sp biomass immobilization with silica gel were performed with identifying functional groups and surface morphology as displayed in Fig. 1 and 2. In Fig. 1, adsorption band at 1087.85 and 964.41 cm-1 show stretching vibration of Si-O-Si and Si-OH, respectively. Existence of water adsorbed was reflected by vibration of νOH at 3444.72 cm-1. Adsorption bands around 746.54 and 470.63 cm-1were produced from vibration of Si-O. Adsorption characteristics on adsorbent resulted from immobilization of Chetoceros sp with silica gel (Fig. 2) were known at band around 2931.8 cm-1, displaying vibration from C-H group coming from Chetoceros sp biomass with silica gel 6 and disappearing several adsorptions coming from functional group on silica. These facts prove that Chetoceros sp biomass exists on silica surface after immobilization process.
SEM analysis result of Chetoceros-silica adsorbent surface (Fig. 2b) shows that surface morphology of Chetoceros-silica adsorbent appears to be brighter than surface morphology of Chetoceros sp without immobilization with silica (Fig. 2a). This fact is caused on Chetoceros-silica adsorbent existing Si atom producing brighter color resulted from higher accelerating of Si atom than Chetoceros sp dominated by organic material.
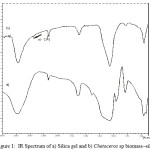 |
Figure 1: IR Spectrum of a) Silica gel and b) Chetoceros sp biomass–silica |
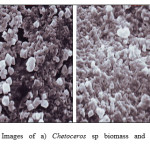 |
Figure 2: SEM Images of a) Chetoceros sp biomass and b) Chetoceros sp biomass–silica |
Adsorption with batch method
Adsorption characteristics from Chetoceros sp biomass adsorbent of immobilization result via encapsulation process with silica aquagel were studied with evaluation of kinetic model, adsorption isotherm, and effect of Pb(II) ion solution pH adsorbeb on the adsorbent. The amount of Pb(II) ion adsorbed q (mg g–1) on adsorbent was calculated using Equation 1:

Where ci and cf are initial and final concentration of Pb(II) ion in solution, v is volume of solution (L), and m is adsorbent mass.
Correlation approach between the amount of Pb(II) ion adsorbed experimentally with batch method and the amount of Pb(II) ion adsorbed estimatically throught kinetic model and adsorption isotherm was determined to know adsorption process optimation with evaluating of kinetic and adsorption isotherm parameters via determining value of the root mean squared error (RMSE) and Chi-square test (χ2) 17, 18 with these equations below:
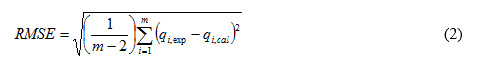

where qi,exp and qi,cal, each was obtained from experimental and estimation result throught adsorption isotherm equation, while m is the number of observation in the experimental isotherm. A smaller RMSE value shows a better curve fitting, moreover, if the data found from the model are near to the experimental results, χ2 can be a small number 17, 19.
Adsorption kinetic
Adsorption equilibrium time needs to be identified to obtain maximum adsorption of adsorbate on adsorbent surface, the shorter the reaction time, the higher the reaction rate. Interaction time additional does not increase the amount of metal adsorbed if equilibrium is reached. Adsorption kinetic is studied with determining adsorption rate constant based on data of time effect to the amount of Pb(II) ion adsorbed on Chetoceros sp encapsulated silica aquagel. In order to investigate the effect of time to adsorption rate of Pb(II) ion on biomass, pseudo first order (Eq. 4) and pseudo second order (Eq. 5) kinetic models may be used 12,20.

Where, adsorption capacities of Pb(II) ions amount at time t and at equilibrium are displayed as qt and qe (mg g–1), the first order and the second order rate constants are described as k1and k2, respectively. Linear relationship of kinetic model both can be seen in Fig. 3 and kinetic parameter data can be found in Table 1.
Table 1: Kinetic parameters of pseudo first order and pseudo second order
|
Kinetic parameters |
R2 |
Adsorption rate |
qe (mmol g–1) |
RMSE |
Χ2 |
|
Chetoceros |
0.991 |
0.032 |
0.0410 |
0.0401 |
0.0009 |
|
Chetoceros–silica |
0.995 |
0.065 |
0.0307 |
0.0309 |
0.0004 |
|
|
|
|
|
|
|
|
Pseudo second order |
|
k2 (g mg–1 min–1) |
|
|
|
|
Chetoceros |
0.923 |
3.139 |
0.0341 |
0.0231 |
0.1049 |
|
Chetoceros–silica |
0.984 |
6.195 |
0.0307 |
0.0269 |
0.0594 |
From the data in Fig. 3 and Table 1, it can be seen that generally, kinetic model of Pb(II) ion on Chetoceros sp biomass and its immobilization result with silica is inclined to follow pseudo first order kinetic model with correlation coefficient value (R2) 0.99 and this is also supported with value of RMSE and Χ2 which are relatively smaller than pseudo second order kinetic model. These cases indicate that only one species is influential to rate adsorption. In these cases, adsorbate or metal ion is as rate determining.
 |
Figure 3: Kinetic linear models of a) pseudo first order and b) pseudo second order on Chetoceros sp biomass and Chetoceros sp–silica |
Adsorption isotherm
Data in Fig. 4 were analyzed with using equations of Freundlich and Langmuir adsorption isotherm (Eq. 5 and 6). Freundlich equation is equation commonly based on heterogeneous surface 21, 22. Common model of Freundlich formula is qe = KfCe1/n, with Kfandn are factor of adsorption capacity and factor of intensity (1–10), respectively. Freundlich linear formula can be described as follow;
![]()
In addition, plot of log qe versus log Cemay result Kf and exponent of n.
In the Langmuir isotherm, a number of active sites which are comparable with surface area is obtained on adsorbent surface. Langmuir formula is displayed as follow;
Ce/qe = 1/qmK + Ce/qm (6)
where, Ce (mg L–1), qe (mg g–1), qm,andK are metal ion solution equilibrium concentration, adsorption capacity of metal ion at equilibrium, adsorption capacity of adsorbent monolayer, and adsorption energy constant, respectively. Furthermore, a straight line for 1/qmas slope and 1/qmK as intercept are obtained from plot log Ce/qe versus Ce. Then, Gibbs free energy formula can be used to calculate adsorption energy;
Adsorption energy = DG0 ads = – R T ln K (7)
With, E, R, T, and K are the adsorption energy (kJ mol–1), the universal gas constant (8.314 J K–1 mol–1), temperature (Kelvin), and the adsorption equilibrium constant, respectively.
Adsorption data of Pb(II) ion on adsorbents found from analysis result calculated from Freundlich and Langmuir formula can be seen in Table 2.
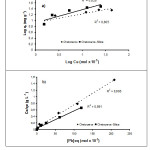 |
Figure 4: Adsorption isotherm linear models of a) Langmuir and b) Freundlich on Chetoceros sp biomass and Chetoceros sp biomass–silica |
If the data in Table 2 is observed, it can be seen that ability of Chetoceros sp adsorbent encapsulated by silica to adsorb metal ions is higher than adsorbent derived from only Chetoceros sp. This indicates that functional groups on Chetoceros sp biomass encapsulated by silica are relatively more active in binding metal ions.
Table 2: Adsorption data of Pb(II) ion on adsorbents
|
Adsorbents |
Langmuir Parameters |
|||||
|
|
|
qm 10–2 ((mol g–1) |
K x 10–2 (mol–1) |
R2 |
RMSE |
Χ2 |
|
Chetoceros |
|
10.286 |
12.111 |
0.990 |
2.468 |
1.579 |
|
Chetoceros–silica |
|
18.868 |
13.874 |
0.999 |
1.404 |
1.696 |
|
|
Freundlich Parameters |
|||||
|
|
|
Kf x 10–2 (mol g–1) |
n |
R2 |
RMSE |
Χ2 |
|
Chetoceros |
|
4.145 |
3.379 |
0.830 |
3.301 |
2.203 |
|
Chetoceros–silica |
|
4.065 |
2.247 |
0.827 |
2.284 |
2.615 |
|
|
|
|
|
|
|
|
|
|
Adsorption energy (∆E) kJ mol–1 |
|||||
|
Chetoceros |
|
11.964 |
|
|
|
|
|
Chetoceros–silica |
|
12.303 |
|
|
|
|
Adsorption energy of Pb(II) metal ion on Chetoceros sp biomass encapsulated by silica aquagel has around 12.303 kJ mol–1. This value of adsorption energy is too small to be as chemical bonding energy but too high to be as physic induction. Therefore, this adsorption can be grouped as weak chemical interaction. This fact is supported by adsorption model following Langmuir model. Langmuir model shows that interaction among metal ions is dominated by chemical bonding and monolayer adsorption.
Interaction pH
pH is one of important factors in controlling adsorption process of metal ion. In this research, influence of pH was studied with varying interaction pH at various pH of 3–8. Influence of pH on Pb(II) metal ion adsorption with Chetoceros sp biomass adsorbent immobilized by silica can be seen in Fig. 5.
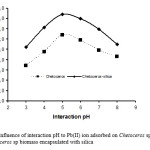 |
Figure 5: Influence of interaction pH to Pb(II) ion adsorbed on Chetoceros sp biomass and Chetoceros sp biomass encapsulated with silica |
Generally, adsorption of Pb(II) metal ion (Fig. 5) has relatively similar model as adsorption increases from pH 3 to optimum pH at around 5 and above pH 5 adsorption starts to decrease. This phenomenon can be explained based on qualitative study of metal ion species existence and adsorbent in solution as pH function. At acid condition, functional groups found on adsorbent may be protonated so that bonding of hydrogen ion (H+) and hydronium ion (H3O+) will occur. At the same time, metal ions in solution before adsorbed by adsorbent hydrolyzes firstly to produce proton.
At pH 5, adsorption is relatively high, this can occur because metal hydroxo complex (PbOH+) formed in solution is much more. In addition adsorbent surface will be negative charge with releasing proton. This case causes increasing of adsorption via electrostatic interaction. At pH > 6, adsorption starts to decline because several species of Pb ion with different charge including Pb(OH)+ and Pb(OH)2 precipitationstart to exist in solution 3.
Conclusions
Immobilization of Chetoceros sp microalgae biomass with silica gel through encapsulation technique has been performed successfully. Adsorption process of Pb(II) ion on Chetoceros sp biomass and Chetoceros sp–silica tends to follow pseudo first order kinetic and Langmuir adsorption isotherm. Adsorbent obtained from immobilization of Chetoceros sp–silica has adsorption rate and capacity to Pb(II) ion in solution higher than Chetoceros sp biomass. In addition, adsorbent immobilized is also more stable chemically as metal ion adsorbent in solution media.
References
- L. Hajiaghababaei, A. Badiei, M.R. Ganjali, S. Heydari, Y. Khaniani, and G.M. Ziarani, Desalination266: 182–187 (2011).
- A. Bhatnagar and A.K. Minocha, Colloids and Surface B: Biointerfaces76, 544−548 (2010).
- V.K. Gupta, S. Agarwal, and T.A. Saleh, J. Hazard. Matter. 185: 17−23 (2011).
- National Library of medicine, Hazardous Substance Data Bank (HSDB), (1996).
- L. Yan-Hui, Z. Yanqiu, Z. Yimin, W. Dehai, and L. Zhaokun, Diam. Relat. Mater. 15: 90−94 (2006).
- Buhani, Suharso and Sumadi, Desalination 259: 140−146 (2010).
- K. Masakorala, A. Turner, M.T. Brown, Environ. Pollut.156: 897−904 (2008).
- P.O. Harris and G.J. Ramelow, Environ. Sci. Technol. 24: 220−228 (1990).
- K. Vijayaraghavan, M. Sathishkumar, and R. Balasubramanian, Desalination 265: 54−59 (2011).
- S. Zakhama, H. Dhaouadi, and F. M`Henni, Bioresource Technol. 102(2): 786−796 (2011).
- V.K. Gupta and A. Rastogi, J. Hazard. Mater. 152: 407−414 (2008).
- Y.G. Bermüdez, I.L.R. Rico, O.G. Bermüdez, and E. Guibal, Chem. Eng. J. 166: 122−131 (2011).
- C. Tong, U.S. Ramellow, and G.J. Ramellow, Intern. J. Environ. Anal. Chem. 56: 175-171 (1994).
- E. Valdman, Bioprocess Eng. 22: 171-173 (2000).M.D. Trevan, Immobilized Enzymes, John Wiley and Sons, New York, 14−15 (1990).
- E.H. Lan, B.C. Dave, J.M. Fikoto, B. Dunn, J.I. Zink, and J.S. Valentine, J. Mater. Chem. 9: 45−53 (1998).
- M.M. Montazer-Rahmati, P. Rabbani, A. Abdolali, A.R. Keshtkar, J. Hazard. Mater.185: 401−407 (2011).
- N. Chen, Z. Zhang, C. Feng, M. Li, D. Zhu, and N. Sugiura, Mater. Chem. Phys. 125: 293−298 (2011).
- L. Vijayaraghavan, T.V.N. Padmesh, K. Palanivelu, and M. Velan, J. Hazard. Mater.B133: 304−308 (2006).
- R. Patel, and S. Suresh, Bioresource Technol.99: 51-58 (2008).
- Y.S. Ho, J.F. Porter, and G. McKay, Water, Air, and Soil Poll.141: 1−33 (2002).
- J. Perić, M. Trgo, and V. Medvidović, Water Res. 38: 1893−1899 (2004).
![]() (4)
(4)

This work is licensed under a Creative Commons Attribution 4.0 International License.









