Effect of Cetyltrimethyl Ammonium Bromide on the Kinetics of Iodination of Aniline by Iodine
Dilip B. Patil1 and Pranita V. Narkhede2
¹Department of Chemistry, Institute of Science, Nagpur-440 001 (India)
²Department of Chemistry, J.D.Polytechnic, Kalmeshwar Road, Nagpur-441501 (India).
Kinetics of the Iodination of aniline by iodine has been studied conventionally. The reaction shows second order kinetics. The effect of various concentration of cetyl trimethyl ammonium bromide has been investigated. It is observed that cetyl trimethyl ammonium bromide inhibits the reaction below critical micelle concentration. The inhibition coefficient has been evaluated and discussed.
KEYWORDS:kinetics; Iodination; cetyltrimethyl ammonium bromide; micelle; Aniline
Download this article as:| Copy the following to cite this article: Patil D. B, Narkhede P. V. Effect of Cetyltrimethyl Ammonium Bromide on the Kinetics of Iodination of Aniline by Iodine. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Patil D. B, Narkhede P. V. Effect of Cetyltrimethyl Ammonium Bromide on the Kinetics of Iodination of Aniline by Iodine. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24034 |
Introduction
The investigation of micellar catalysis has provoked a great interest during last few decades, since the study of micelle catalyzed reaction might provide a basic interpretation of some aspect of catalysis.1-4 The continuing research interest in the area of micelle formation, structure and their role in reaction catalysis reflects the importance of the subject. The extensive study and publications in the book summarize much of the knowledge about micelles.5-14
On the other hand, there are certain cationic surfactant like cetyltrimethyl ammonium bromide, that can affect the rate of aroniatic substitution reactions and plays important role in chemistry due to their ability to catalyze or inhibit certain reactions.15-17
The purpose of this paper is to show the effect of cetyl trimethyl ammonium bromide (CTAB) on the kinetics of iodination of aniline by iodine.
Experimental
Preparation of Solutions
The solutions were prepared for each set of experiments. Doubly distilled water was used for preparing various solutions of 5.0 x 10-2 M iodine and aniline were prepared along with 0.1 M cetyl trimethyl ammonium bromide and 0.1 M sodium iodide.
Critical Micelle Concentration
Critical micelle concentration of cetyltrimethyl ammonium bromide was determined by measuring absorbance of acridine orange (1.0 x 10-3 M) in various concentrations of CTAB ranging from 6.0 x 10-3 M to 13.0 x 10-3 M.
Kinetic Study
The rate of reaction is followed by determining the unreacted iodine from the iodine-aniline (5.0 x 10-3 M each) system at various time intervals. The reciprocal of concentration of unreacted iodine was plotted versus time. (Table 1 and Fig. 1) Similar rate determinations were made in presence of various concentrations of cetyl trimethyl ammonium bromide and the ploto of unreacted iodine versus time was plotted (Fig. 2).
Results And Discussion
In order to study the kinetics of Iodination of aniline by iodine, equally concentrated aniline and iodine were mixed together. The final concentration of each reactant was 5.0 x 10-3 M. The unreacted iodine was titrated against 2.5 x 10-3 M sodium thiosulphate using freshly prepared starch as an indicator. Reciprocal of unreacted iodine was plotted versus time. A straight line graph was obtained (Table 1 and Fig. 1). The second order rate constant evaluated from this graph is 0.026 M-1 S-1.
In the following stages of the study, the effect of micellar media on the rate of reaction was investigated. For this purpose, critical micelle concentration of cetyl trimethyl ammonium bromide (CTAB) was determined in presence of equally concentrated aniline and iodine : The CMC of CTAB in presence of 5.0 x 10-3 M aniline and iodine was found out to be 1.0 x 10-3 M from the absorption versus concentration of CTAB graph using acridine orange as colouring agent.
The 1/[I2] versus time graph (Fig. 2) was plotted with the observed unreacted iodine values during the reaction of 5.0 x 10-3 M aniline and iodine solutions in the presence of CTAB in different concentrations in order to see its effect on second order kinetics. The second order rate constants were evaluated. The dependence of these rate constants on CTAB concentration has been shown in Table 2 and Fig. 3.
The critical micelle concentration of CTAB does not affected by 5.0 x 10-3 M aniline and iodine. It indicates that iodine solubilization do not occur within the micelles. Hence the tri-iodide formation in presence of iodide ion is resonable to consider, since cetyl trimethyl ammonium bromide favours the formation of tri-iodide ion and stabalize it.
Fig. 2 shows that the presence of CTAB in the reaction medium, though at much lower concentration than critical micelle concentration, leads to the inhibition of the iodination of aniline by iodine. Fig. 2 and Fig. 3 leads us to conclude that the iodination of aniline is inhibited by the cetyl trimethyl ammonium bromide.
Concentration of Aniline: 5.0 x 10-3 M
Concentration of Iodine: 5.0 x 10-3 M
Concentration of Sodium iodide: 5.0 x 10-2 M
Concentration of sodium thiosulphate: 2.5 x 10-2 M
Volume of reaction mixture titrated
for each estimation of unreacted
iodine: 25.0 cm3
Temperature: 25.0ºc
Table 1 : Kinetics of Iodination of Aniline by Iodine
|
Time/s |
Volume of thiosulphate.cm3 |
Unreacted Iodine, [I2]/10-3 M |
I/[I2]/M-1 |
|
0 |
16.0 |
5.00 |
200 |
|
1200 |
13.9 |
4.35 |
230 |
|
2400 |
12.3 |
3.85 |
260 |
|
3600 |
10.9 |
3.42 |
292 |
|
4800 |
9.9 |
3.09 |
324 |
|
6000 |
9.0 |
2.81 |
356 |
|
7200 |
8.3 |
2.60 |
385 |
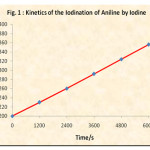 |
Figure 1: Kinetic of the Iodination of Aniline by Iodine |
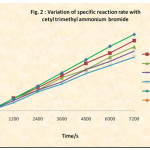 |
Figure 2: Variation of Specific Reaction Rate with Cetyl Trimethyl Ammoniun Bromide |
Concentration of Aniline : 5.0 x 10-3 M
Concentration of Iodine : 5.0 x 10-3 M
Concentration of Sodium iodide : 5.0 x 10-2 M
Temperature : 25.0ºc
Table 2 : Kinetics of Iodination of Aniline by Iodine : in presence of CTAB
|
[CTAB]/10-6 M |
Specific reaction rate k2/10-2 M-1 S-1 |
|
– |
2.6 |
|
1.0 |
2.4 |
|
2.0 |
2.2 |
|
3.0 |
2.0 |
|
4.0 |
1.8 |
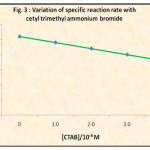 |
Figure 3: Variation of Specific Reaction Rate With Cethyl Trimethyl Ammonium Bromide |
References
- E. J. Fendler and J. H. Fendler, Advances in Phys. Org. Chem; 8, 271 (1970).
- J. H. Fendler and E. J. Fendler; “Catalysis in micellar and macromolecular systems”, Academic press, New York, (1975).
- E. H. Cordes, “Reaction kinetics in micelles,” Plenum pub; New York, (1973).
- K. L. Mittal; “Micellizaztion, solublization and microemulsion,” Plenum Press, New York, Vol. 2 (1977).
- E. J. Fendler and J. H. Fendler, Advances in Phys. Org. Chem; 8, 271 (1970).
- E. H. Cordes, “Reaction kinetics in micelles,” Plenum pub; New York, (1973).
- D. Tanford, “The hydrophobic effect,” John Wiley & Sons, New York, (1973).
- J. H. Fendler and E. J. Fendler; “Catalysis in micellar and macromolecular systems”, Academic press, New York, (1975).
- L. R. Fisher and D. G. Oakenfull; Chem. Soc. Rev., 6, 25, 1977.
- P. Mukherjee, Ber. Bunsenges Phys Chem., 82, 931 (1978).
- F. M. Menger; Accounts of Chem. Research, 12, 111 (1979).
- E. A. G. Aniansen; S. N. Wall; M. Almgren, H. Hoffman, I. Kielmann, W. Ulbricht, R. Zora, J. Lang and C. Tondre, J. Phys. Chem., 80, 905 (1976).
- F. M. Menger, J. M. Jerkunica and J. C. Johnstan, J. Am. Chem. Soc. 100, 4676 (1978).
- P. Mukherjee and J. R. Cardinal; J. Phys. Chem; 82, 1620 (1978).
- D. V. Griffiths and M. L. Bender; Advances in catalysis, 23, 203 (1973).
- R. J. Bergeron; J. Chem. Educ., 54, 204 (1977).
- M. L. Bender and M. Komiyama; “Cyclodextrin chemistry”, Spingerverlag, Berlin (1978).

This work is licensed under a Creative Commons Attribution 4.0 International License.









