Determination of Abamectin by Dispersive Liquid-liquid Microextraction Combined with High-Performance Liquid Chromatography in Fruit Juice Samples
Mohammad Bagher Pashazanousi1*, Hossein Ali Mashayekhi2 and Mohammad Rezaee3
1Department of Chemistry, Chalous Branch, Islamic Azad University, Chalous (Iran).
2Department of Chemistry, Tonekabon Branch, Islamic Azad University, Tonekabon (Iran).
3Nuclear Fuel Cycle Research School, Nuclear Science and Technology Research Institute, Atomic Energy Organization of Iran, P.O. Box 14395-836, Tehran (Iran).
Dispersive liquid-liquid microextraction (DLLME) coupled with high-performance liquid chromatography (HPLC) – UV detection was applied for the extraction and determination of abamectin (avermectin (B1b) and avermectin (B1a)) in fruit juice samples. An appropriate mixture of ethanol (as the disperser solvent) and carbon tetrachloride (as the extraction solvent) was injected rapidly into a sample containing abamectin. After extraction, phase separation was performed by centrifuging the mixture and the sedimented phase was analyzed by HPLC-UV. Some effective parameters on the extraction, such as types and volumes of extractant and disperser solvents and salt effect were investigated and optimized. Under the optimum conditions, (extractant solvent: carbon tetrachloride, 30.0 µL; disperser solvent: ethanol, 1.0 mL and without salt addition), the calibration graphs were linear in the range of 2.5 - 500 µg L-1 and 1.0 – 500 µg L-1 with the detection limits of 0.8 µg L-1 and 0.3 µg L-1 for B1b and B1a in fruit juice samples, respectively. The relative standard deviation (R.S.D., n = 5) for the extraction and determination of 50.0 µg L-1 of B1b and B1a in the fruit juice samples were 9.2 and 7.8%, respectively. The results show that DLLME is a very simple, rapid, sensitive and efficient analytical method for the determination of abamectin in fruit juice samples and satisfactory results were obtained.
KEYWORDS:Dispersive liquid-liquid microextraction; Abamectin; High-performance liquid chromatography; Fruit juice samples
Download this article as:| Copy the following to cite this article: Pashazanousi M. B, Mashayekhi H. A, Rezaee M. Determination of Abamectin by Dispersive Liquid-liquid Microextraction Combined with High-Performance Liquid Chromatography in Fruit Juice Samples. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Pashazanousi M. B, Mashayekhi H. A, Rezaee M. Determination of Abamectin by Dispersive Liquid-liquid Microextraction Combined with High-Performance Liquid Chromatography in Fruit Juice Samples. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23827 |
Introduction
The use of pesticides in modern agricultural practices is a major issue for the protection of crops against pests and diseases. Event though, several reasons for not using pesticides have come out as a result of their undiscrimate application: i.e. (1) they do not completely solve pest problems, (2) they are hazardous to human health (especially in children) and animals, (3) they often contaminate food, water and air, (4) there is much unknown information about their behavior in the environment, in the human body, etc… In spite of the inconveniences of their use, it is fact very difficult to do without them in the current state of the world agriculture. Abamectin, which belongs to the macrocyclic lactone class of avermectins and is comprised of at least 80% of avermectin B1a and less than 20% of avermectin B1b (Fig. 1), is produced by the soil bacterium streptomyces avermitilis.1,2 Because of its high toxicity to agriculture pests by acting on nervous system, abamectin is widely used to control insects and mites in vegetables and fruits.3-5
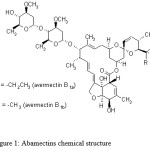 |
Figure 1: Abamectins chemical structure |
Abamectin in various biological matrices including animal tissues, fruit juice, and vegetables can be successfully determined by high-performance liquid chromatography (HPLC) in the reverse-phase mode, coupling with different detection modes, such as ultraviolet detection (UVD), fluorescence detection (FLD), and mass spectrometry.6-8 To eliminate interferences from the biological matrices, liquid-liquid extraction (LLE) and solid-phase extraction (SPE) have been proposed for the clean up step frequently.9,10 LLE involves multiple time-consuming steps and requires large volumes of organic solvent which costs high and is dangerous to analytes. Although SPE requires less solvent and shorter preparation time than LLE, the SPE and cartridge are also rather expensive. Both above processes involve manipulation of samples, and therefore, they will be subject to human errors. In order words, any clean-up procedure may cause partial loss of analyte and consumption of labor, time, and cost.
Recently, Rezaee et al. have introduced a novel modality of liquid–liquid microextraction, referred as dispersive liquid–liquid microextraction (DLLME).11 DLLME employs a mixture of a high–density solvent extractant and a water miscible, polar solvent as the disperser. Acetone, methanol and acetonitrile can be used as disperser solvents; whereas chlorinated solvents (e.g. chlorobenzene and chloroform) are useful as extractants. When this solution is added to a sample forming a cloudy state that consisting fine droplets of extractants dispersed in the matrix. The large contact surface between the sample and the droplets speeds up the mass transference processes. After centrifugation, the extractant solvent settles on the bottom of the vial. Up to now, DLLME has been successfully applied for extraction of organic and inorganic analytes.12-23
In the present study, the applicability of the DLLME method combined with HPLC-UV for the determination of abamectin in fruit juice samples was investigated. The effects of various experimental parameters such as types and volumes of extraction and disperser solvents and salt addition on the extraction efficiency were studied. Further, the performance of the method for analysis of real samples was studied.
Experimental
Chemicals and reagents
Pesticides standard of abamectin purchased from Sigma-Aldrich Company was of analytical standard with purity of > 99%. Standard solutions of abamectin were prepared in acetonitrile (1000 mg L-1) and kept in the dark under refrigeration at 4 ºC. Working mixtures of pertinent concentrations were prepared daily by appropriate combination and dilution. All different fruit juice samples (Orange and Kiwi) were prepared by fruits which produced from local garden fruits (Tonekabon, Iran). Carbon tetrachloride (GR), carbon tetrachloroethylene, chloroform, dichloromethane and 1,2-dichloroethane as extraction solvents were obtained from Merck Company (Germany). Acetone, acetonitrile, ethanol and methanol as dispersive solvents were obtained from Merck. Also, sodium chloride was purchased from Merck. The water used was purified on a youngling ultra pure water purification system (aqua maxTM – ultra, korea).
HPLC system
Chromatographic separations were carried out on a Waters Breeze with 1525 Binary HPLC pump (Massachusetts, USA) and having a 20 µL sample loop and equipped with a Waters 2487 UV/Vis detector. Separations were carried out on a Waters Spherisorb ODS2 column (250mm × 4.6 mm, with 5.0 µm particle size) from Waters Company (Massachusetts, USA). A mixture of water and methanol (8:92) at a flow rate of 1.5 mL min-1 was used as a mobile phase in isocratic elution mode. The injection volume was 20 µL for all the samples and the detection was performed at the wavelength of 245 nm.
Dispersive liquid-liquid microextraction procedure
A 5.0 mL of doubly-distilled water was placed in a 10-mL glass tube with conical bottom and spiked at level of 100.0 µg L-1 of analytes. Ethanol (1.0 mL), as disperser solvent, contains 30.0 µL carbon tetrachloride, as extraction solvent was injected, rapidly into the sample solution by using a syringe. The cloudy solution produced was centrifuged for 3min at 6000rpm. After centrifuging, the dispersed fine droplets of carbon tetrachloride sedimented in the bottom of test tube (about 20.0 ± 1.0 µL). The sedimented phase was completely transferred to another test tube with conical bottom using 50 µL HPLC syringe and after evaporation of the solvent in a water bath; the residue was dissolved in 20 µL HPLC grade methanol and injected into the separation system. All experiments were performed in duplicate and means of results were used in plotting of curves or in tables.
Results and Discussion
In the present research, the DLLME combined with HPLC-UV was applied for determination of avermectin (B1b) and avermectin (B1a) in fruit juice samples. In order to obtain a high recovery and enrichment factor, the effect of different parameters such as kind and volumes of the extractant and disperser solvents and salt addition on the extraction efficiency of B1b and B1a were examined and optimized. Enrichment factor (EF) and percent recovery (R %) as analytical responses were calculated based on the following equations:
EF = Csed/C0 (1)
![]()
Where, EF, Csed and C0 are the enrichment factor, concentration of the analyte in the sedimented phase and initial concentration of the analyte in the sample, respectively. R%, Vsed and Vaq are the percent recovery, volume of the sedimented phase and volume of the sample, respectively. Csed is calculated from a calibration curve which was obtained by direct injection of abamectin with the concentrations in the range of 5 -100 mg L-1.
Selection of extraction solvent
The selection of an appropriate solvent is a major parameter for DLLME process. Organic solvents are selected based on their higher density rather than water, extraction capability of the interested compounds. In the present study, carbon tetrachloroethylene, carbon tetrachloride, dichloromethane, 1,2-dichloroethane and chloroform were selected as extractant solvents. The study was performed by using 1.0 mL of ethanol containing different volumes of the extractant solvent to produce about 20.0 µL of the sedimented phase. Thereby, 26.0, 30.0, 102.0, 65.0 and 60.0 µL of carbon tetrachloroethylene, carbon tetrachloride, dichloromethane, 1,2-dichloroethane and chloroform were used, respectively. Average percent recoveries for different extractant solvents are shown in Table 1. The results revealed that carbon tetrachloride has the highest extraction recovery in comparison with the other tested solvents. It is probably because of higher solubility of abamectin in carbon tetrachloride in comparison with other tested solvents. Therefore, carbon tetrachloride was selected as the extraction solvent.
Table 1: Extraction recovery of different extraction solvents evaluated for the extraction of abamectin (B1b and B1a)a
|
Extraction recovery (%) |
Compounds |
||||
|
Dichloromethane |
Carbon tetrachloroethylene |
1,2-dichloroethane |
Carbon tetrachloride |
Chloroform |
|
|
37.3 |
60.0 |
55.3 |
75.3 |
53.3 |
B1b |
|
34.7 |
52 |
56.0 |
71.3 |
58.0 |
B1a |
Selection of disperser solvent
Miscibility of disperser solvent in organic phase (extraction solvent) and aqueous phase (sample solution) is the most important point for selection of disperser solvent. Thereby, acetone, acetonitrile, ethanol and methanol which have this ability, are selected for this purpose. A series of sample solution were studied by using 1.0 mL of each disperser solvent containing 30.0 µL carbon tetrachloride (as extraction solvent). The results illustrated in Table 2. According to the results, variations of extraction recoveries by using different disperser solvents are not remarkable, thus, ethanol is selected because of low toxicity and having a little higher extraction recovery.
Table 2: Extraction recovery of different disperser solvents evaluated for extraction of abamectin (B1b and B1a)a
|
Extraction recovery (%) |
Compounds |
|||
|
Ethanol |
Methanol |
Acetonitrile |
Acetone |
|
|
76 |
74 |
73 |
74 |
B1b |
|
72 |
70 |
71 |
69 |
B1a |
aExtraction conditions: sample volume, 5.0 mL; disperser solvent (acetone, acetonitrile, ethanol and methanol) volume, 1.0 mL; extraction solvent (carbon tetrachloride) volume, 30.0 µL; concentration of analytes, 100µg L-1.
Effect of extraction solvent volume
To examine the effect of extractant solvent volume on the extraction efficiency, solutions containing different volumes of carbon tetrachloride (30.0, 45.0, 60.0, 75.0 and 90.0 µL) and fixed volume of ethanol (1.0 mL) were used for DLLME procedures. It is clear that by increasing the volume of carbon tetrachloride from 30.0 to 90.0 µL, the volume of the sedimented phase increases from 20.0 to 77.0 µL. On the other hand, enrichment factor decreases by increasing of the volume of carbon tetrachloride (Fig. 2) due to increasing of the sedimented phase volume. The volume of extractant solvent has to be selected to obtain high EF. In the following studies, 30.0 µL of carbon tetrachloride was selected as an optimal volume of the extractant solvent.
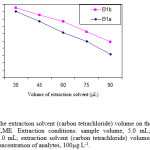 |
Figure 2: Effect of the extraction solvent (carbon tetrachloride) volume on the enrichment factor obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; disperser solvent (ethanol) volume, 1.0 mL; extraction solvent (carbon tetrachloride) volumes, 30.0, 45.0, 60.0, 75.0 and 90.0 µL; concentration of analytes, 100µg L-1. |
Effect of disperser solvent volume
Variation of the volume of ethanol (as disperser solvent) causes changes in the volume of sedimented phase. To obtain a constant volume of sedimented phase, the volumes of ethanol and carbon tetrachloride were changed, simultaneously. The experimental conditions were fixed and included the use of different volumes of ethanol, (0.50, 1.0, 1.5 and 2.0 mL) containing 24.0, 30.0, 37.0 and 42.0 µL of carbon tetrachloride, respectively. The results are shown in Fig. 3. Accordingly, the extraction efficiency of abamectin increases by increasing of the volume of ethanol and then decreases by further increasing of the volume of ethanol. It seems that, in the low volume of ethanol, a cloudy state is not formed well, thereby, the recovery is low. In higher volumes of ethanol, solubility of abamectin in aqueous solutions increases. Therefore, the extraction efficiency decreases due to the decrease of distribution coefficient. A 1.0 mL of ethanol was chosen as optimum volume.
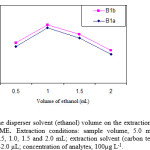 |
Figure.3: Effect of the disperser solvent (ethanol)volume on the extraction recovery of analytes obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; disperser solvent (ethanol) volumes, 0.5, 1.0, 1.5 and 2.0 mL; extraction solvent (carbon tetrachloride) volumes, 24.0, 30.0, 37.0 and 42.0 µL; concentration of analytes, 100µg L-1. |
Effect of extraction time
In DLLME, extraction time is defined as interval time between injection the mixture of disperser solvent (ethanol) and extraction solvent (carbon tetrachloride), before starting to centrifuge. According to the other papers,12-23 time has no influence on extraction efficiency, because of that after formation of cloudy solution, the surface area between extraction solvent and aqueous phase (sample) is infinitely large. Thereby, transition of analytes from aqueous phase (sample) to extraction solvent is fast. Subsequently, equilibrium state is achieved quickly so the extraction time is very short. This is the most advantage of DLLME technique which is time independence. In this method, time-consuming step is centrifuging of sample solution in extraction procedure, which is about 3 min.
Salt addition
The effect of salt addition on the extraction efficiency of abamectin was evaluated by adding of NaCl (0-8%, w/v) into the sample solution containing 100 µg L-1 of abamectin and applying the DLLME procedure. By increasing of NaCl %, the volume of sedimented phase increases, because of the decrease in solubility of the extractant solvent in the presence of salt. Figure 4 show that enrichment factor decreases in the presence of salt; because of increasing in the volume of the sedimented phase. Therefore, further experiments were done without salt addition.
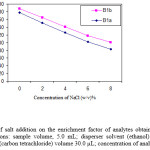 |
Figure 4: Effect of salt addition on the enrichment factor of analytes obtained from DLLME. Extraction conditions: sample volume, 5.0 mL; disperser solvent (ethanol) volume, 1.0 mL; extraction solvent (carbon tetrachloride) volume 30.0 µL; concentration of analytes, 100 µg L-1. |
Analytical performance
Figures of merit of the proposed method are tabulated in Table 3. Calibration curves were obtained under the optimized conditions with linear dynamic range of 2.5 – 500 µg L-1 and 1.0 – 500 µg L-1 for B1b and B1a in fruit juice samples, respectively. The enrichment factors of the method were 171 and 163 for B1b and B1a in fruit juice samples, respectively, at the concentration level of 100 µg L-1 of analytes. The relative standard deviations (RSDs, n = 5) at the concentration level of 50.0 µg L-1 were 9.2 and 7.8% for B1b and B1a, in fruit juice samples, respectively. The limit of detections (LODs) based on signal-to-noise ratio (S/N) of 3 were 0.8 µg L-1 and 0.3 µg L-1 for B1b and B1a in fruit juice samples, respectively.
Table 3: Quantitative results of DLLME and HPLC-UV of abamectin (B1b and B1a)a
|
R2d |
R.S.D. (%)c |
EFb |
LODa (µg/L) |
Linear range (µg/L)
|
Sample
|
||||||||||||
|
B1a |
B1b |
B1a |
B1b |
B1a |
B1b |
B1a |
B1b |
B1a |
B1b |
||||||||
|
0.998 |
0.997 |
6.5 |
7.3 |
180 |
190 |
0.1 |
0.5 |
0.5-500 |
1.0-500 |
Water |
|||||||
|
0.996 |
0.995 |
7.8 |
9.2 |
163 |
171 |
0.3 |
0.8 |
1.0-500 |
2.5-500 |
Fruit juice (orange) |
|||||||
Extraction of the abamectin in fruit juice samples
Due to the importance of analysis of abamectin in fruit juice samples, the proposed method was applied to determine the concentration of the analytes in the fruit juice samples (Orange and Kiwi), and the obtained results are summarized in table 4. In order to reduce the matrix effect, the fruit juice samples were diluted to 1:5, using deionized water. The results showed that both samples were free from abamectin contamination. Thus, they were spiked with abamectin standards to assess matrix effects. Figure 5 show the chromatograms obtained for the fruit juice sample before and after spiking with concentration of abamectin (5.0 µg L-1). Also, the results of relative recoveries of the fruit juice samples are tabulated in Table 4. The data in Table 4 show that the relative recoveries of abamectin were in the ranges of 92% to 96%, demonstrating that the fruit juice samples matrices had little effect on the DLLME.
 |
Figure 5: HPLC chromatograms of (a) before spiking with analytes in fruit juice (Orange), (b) 5.0 μg L-1 spiked of analytes in fruit juice after extraction via proposed method at optimum conditions. Extraction conditions, as with Fig.4. |
Table 4: Determination of abamectin (B1b and B1a) in fruit juice samples and relative recovery of spiked B1b and B1a in fruit juice samples
|
Relative recovery (%) |
Found B1b and B1a ( µg L-1) ± SD , n=3 |
Added of B1b and B1a ( µg L-1) |
Concentration of B1b and B1a ( µg L-1) |
Sample |
|||||
|
B1a |
B1b |
B1a |
B1b |
B1a |
B1b |
B1a |
B1b |
|
|
|
96 |
94 |
4.8 ± 0.5 |
4.7 ± 0.4 |
5.0 |
5.0 |
n.da. |
n.da. |
Fruit juice (Orange) |
|
|
92 |
90 |
4.6 ± 0.3 |
4.5 ± 0.4 |
5.0 |
5.0 |
n.d. |
n.d. |
Fruit juice (Kiwi) |
|
Conclusions
This paper describes the application of the DLLME method combined with HPLC-UV, for the determination of abamectin (B1b and B1a) in fruit juice samples. The results demonstrated that this combined method produced acceptable relative recoveries for abamectin in fruit juice samples.
In DLLME method, sample preparation time and consumption of toxic organic solvents are minimum. Further, our finding results in the present study show that, our proposed method (DLLME), has lower LOD and much shorter extraction time for the determination of abamectin in fruit juice samples. This method is also simple, fast and inexpensive.
Acknowledgement
Financial support from Department of Chemistry, Chalous Branch, Islamic Azad University (Chalous, Iran) for the support during the period of this research is gratefully acknowledged.
References
- Sun, Y.J., Diao, X.P., Zhang, Q.D. and Shen, J.Z. Chemosphere 60:699 (2005).
- Campbell, W.C., Fisher, M.H., Stapley, E.O., Albers-Schonberg, G. and Jacob, T.A. Science 221:823 (1983).
- Huang, J. and Casida, J.E. J. Pharmcol Exp Ther 281:261 (1997).
- Wang, Q., Chen, J.A., Liu, Z.M., Wu, S.G., Zho, X.P. and Wu, C.X. Insect Sci 12:109 (2005).
- Ship, J.L., Wang, K. and Ferguson, G. Biol Control 17:125 (2000).
- Valenzuela, A.I., Popa D.S. and Redondo, M.J. J. Chromatogr. A 918:59 (2001).
- Roudant, B. Analyst 123:2541 (1998).
- Kolar, L., Kuzjner, J. and Erzjen, N.K. Biomed Chromatogr 18 :117 (2004).
- Bienvenida, G.L., Garcia-Reyes, J.F. and Antonio, M.D. Talanta 79:109 (2009).
- Feas, X., Seijas, J.A., Vazquez-Tato, M.P., Regal, P., Cepeda, A. and Fente, C. Anal. Chim. Acta 631:237 (2009).
- Rezaee, M., Assadi, Y., Milani Hosseini, M.R., Aghaee, E., Ahmadi, F. and Berijani, S. J. Chromatogr. A 1116:1 (2006)
- Jiang, H., Qin, Y. and Hu, B. Talanta 74:1160 (2008).
- Liang, P., Xu, J. and Li, Q. Anal. Chim. Acta 609:53 (2008).
- Lopez, M.G., Rodriguez, I. and Cela, R. J. Chromatogr. A 1166:9 (2007) .
- Shamsipur, M. and Ramezani, M. Talanta 75:294 (2008).
- Farina, L., Boido, E., Carrau, F. and Dellacassa, E. J. Chromatogr A 1157:46 (2007) .
- Rezaee, M., Yamini, Y. and Faraji, M. J. Chromatogr. A 1217:2342 (2010).
- Rezaee, M., Yamini, Y., Shariati, S., Esrafili, A. and Shamsipur, M. J. Chromatogr A 1216:1511 (2009).
- Mashayekhi, H.A., Abroomand-Azar, P., Saber-Tehrani, M. and Waqif, S.H. Chromatographia 71:517 (2010).
- Rezaee, M., Yamini, Y., Mashayekhi, H.A., Naeeni, M.H., and Bashiri Jubari, M. J. Chin. Chem. Soc. 58:332 (2011).
- Moghimi, A. J. Chin. Chem. Soc. 55:369 (2008).
- Farhadi, K., Maleki, R. and Mohammad Nezhad N. J. Chin. Chem. Soc. 56:575 (2009).
- Mashayekhi, H.A., Abroomand-Azar, P., Saber-Tehrani, M. and Waqif, S.H. Int. J. Environ. Anal. Chem. 91:516 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









