Corrosion Inhibition of Cefuroxime Axetil on Mild Steel with Acid Solution
N. Banumathi* and N. Kasthuri
Department of Chemistry, SriGuru Institute of Technology, Saravanampatti, Coimbatore 641 110 (India).
The inhibition of corrosion on mild steel in 1M H2SO4 by a heterocyclic compound namely cefuroxime axetil has been studied by weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy methods. The adsorption characters of the inhibitor on mild steel, free energy change and energy of activation of the inhibition process have been calculated from the results. The inhibition efficiency increases with inhibitor concentration and decreases with temperature. The adsorption of the inhibitor on the mild steel surface obeys Langmuir adsorption isotherm. Potentiodynamic polarization studies show that the inhibitor behaves as mixed type. Addition of halide ions enhances the inhibition efficiency.
KEYWORDS:Corrosion inhibition; mild steel; Cefuroxime axetil; Inhibition efficiency; Langmuir adsorption
Download this article as:| Copy the following to cite this article: Banumathi N, Kasthuri N. Corrosion Inhibition of Cefuroxime Axetil on Mild Steel with Acid Solution. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Banumathi N, Kasthuri N. Corrosion Inhibition of Cefuroxime Axetil on Mild Steel with Acid Solution. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24007 |
Introduction
Mild steel is one of the alloy which find applications for making the entire machinery in industries and for construction of industrial buildings. The surface of the mild steel must be freed from oxide by acid pickling and subjected to surface treatment such as painting enamelling etc. Organic heterocyclic compounds containing azole nucleus have been found to be effective inhibitors for steel in different corrosive media1-3. Some of the organic compounds reported as inhibitors for mild steel in acid media were pyrrolidine dithiocarbomate4, beta phenylethylamine5, 5-hydroxyindole and nitroindole6-8, triazole derivatives9-12, substituted dithiobiurets13, tetrazine derivatives14, hydrazine and substituted hydrazines15, quinoline derivatives16, thiourea derivatives17, pyridine derivatives18.In this part of the present work, corrosion of mild steel in 1M H2SO4 ,the effect of cefuroxime–axetil as inhibitor in combating the corrosion have been investigated.
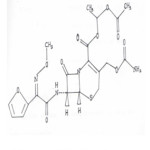 |
Figure 1: Structure of cefuroxime axetil Click here to View figure |
Experimental Procedure
The mild steel specimens of the following composition have been used for weight loss, polarization and impedance studies.Carbon:0.06,Sulphur:Nil, Phosphorous:0.009, Silicon: Nil, Manganese:0.32. The sulphuric acid used is of A.R.grade for the preparation of 1M H2SO4 solution using double distilled water. The inhibitor used is cefuroxime axetil prepared by dissolving in acetone. The inhibitor is available as an anti –inflammatory drug. Mild steel panel of 1.5 cm x 2.5 cm has been used for weight loss measurements, while for the polarization experiments a cylindrical rod embedded in teflon with an exposed area of 0.5 cm x 0.5 cm was used. Before each experiment the electrodes were polished with different grade emery papers, degreased with tetrachloroethylene rinsed under running water and finally dried.
Weight loss method
The polished and pre-weighed mild steel specimen were carefully hanged in hooks and suspended in 100 mL test solutions with and without inhibitors of different concentration at 298 K. The temperature was controlled by an aqueous thermostat. After 3 h of immersion in the corrodent solution, the specimen was carefully washed in double distilled water dried and then weighed. The percentage of inhibition efficiency (IE%) for various concentrations of the inhibitor were calculated as
Inhibitation Efficiency%= ![]()
where = weight loss without inhibitor andweight loss with inhibitor
Temperature studies
The effect of temperature on the inhibitory action of the inhibitor was determined by weight loss method with various concentrations at different temperature (308,313 & 318 K) for a fixed immersion time of 1 h.
Synergistic effects of halide ions
The synergistic effect was studied in the presence of 2 mM KCl and KI to the steel specimen immersed for 3 hours in 1 M sulphuric acid containing 2 mM concentration of the inhibitor. The same weight loss method procedure has been followed to study the synergistic effect. From the weight loss data, the corrosion rate and the inhibition efficiency were calculated.
Polarisation and impedance studies
Electrochemical studies were carried out using the potentiostat model 1280 B solartron (U.K). They were carried out in a glass cell with a capacity of 100 mL. A platinum electrode and a saturated calomel electrode (SCE) were used as counter electrode and reference electrode respectively. The polarisation measurements were carried out with a scan rate of 1 mV/Sec in the range of -200 mV to +200 mV vs corrosion potential of the working electrode measured against SCE. The AC impedence measurements are shown as Nyquist plots and polarisation data as Tafel plots. The impedence measurements were made at a corrosion potentials with the Ac voltage amplitude 10 mV in the frequency range 10 KHz to 10 MHz. The Icorr, Ecorr, Rt and Cdl values were obtained from the data using the corresponding “corr view” and “Z view” softwares.
Results and Discussion
Weight loss studies
The cefuroxime axetil was tested for six different concentrations (0.001mM to 2.0mM).The corrosion rate and inhibition efficiency calculated based on weight loss data are given in Table 1. Table 1 reveals that inhibition efficiency increase with an increase in the concentration of the inhibitor reaching a maximum of ≈79 to 97 % at 2mM (Fig.1). At the same time the corrosion rate decreases with increase in inhibitor concentration. The increase in inhibition efficiency with increase in concentration may be attributed to the increase in surface coverage (θ) by the adsorption of inhibitor on the steel surface.
Table 1: Inhibition efficiency of cefuroxime axetil on mild steel in 1M H2SO4
|
S.No |
Inhibtor concentration (mM) |
Weight loss (g) |
Corrosion rate (mmpy) |
Inhibitor efficiency (%) |
Surface coverage (θ) |
|
1 |
Blank |
0.1776 |
270.31 |
– |
– |
|
2 |
0.001 |
0.0341 |
51.90 |
79.4 |
0.7940 |
|
3 |
0.01 |
0.0207 |
31.50 |
87.5 |
0.8750 |
|
4 |
0.1 |
0.0187 |
28.46 |
88.7 |
0.8870 |
|
5 |
1.00 |
0.0110 |
16.74 |
93.0 |
0.9300 |
|
6 |
2.0 |
0.0066 |
10.04 |
97.0 |
0.9700 |
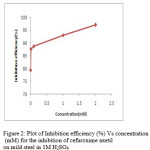 |
Figure 2: Plot of Inhibition efficiency (%) Vs concentration (mM) for the inhibition of cefuroxime axetil on mild steel in 1M H2SO4 Click here to View figure |
Effect of Temperature
To investigate the mechanism of inhibition and to determine the activation energies of the corrosion process, the weight loss studies were carried out at higher temperatures from 308-318 K. Table 2 reveals that the rate of corrosion of mild steel increases with increase in temperature in the presence of the inhibitor.
It is observed that inhibition efficiency decreases with increase in temperature from 308-318 K. The decrease in inhibition efficiency with temperature may be attributed to desorption of the inhibitor molecules from metal surface at higher temperature 19.
Table 2: Inhibition efficiency (%) of 2mM concentration of cefuroxime axetil and corrosion rate of mild steel in corrosion in 1 M H2SO4 at different temperature
|
S.No |
Temperature (K) |
Weight loss (g) |
Corrosion rate (mmpy) |
IE(%) |
|
1 |
308 |
0.0208 |
31.65 |
88.49 |
|
2 |
313 |
0.0335 |
50.98 |
81.79 |
|
3 |
318 |
0.0534 |
81.27 |
72.16 |
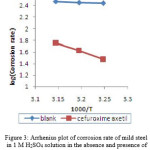 |
Figure 3: Arrhenius plot of corrosion rate of mild steel in 1 M H2SO4 solution in the absence and presence of 1 mM concentration of the cefuroxime axetil. Click here to View figure |
Fig. 1 presents the Arrhenius plots of log corrosion rate vs. 1/T for 1 M H2SO4 with and without the addition of the inhibitor and the slopes of the straight lines permit the calculation of Arrhenius activation energy, Ea. The value of Ea (76.80kJ/mol) increases in the presence of inhibitor. Szauer, Brandt20 and Foroulis21 proposed that the lower activation energy value of the process in the presence of the inhibitor compared to that in its absence is attributed to chemisorptions, while the opposite is attributed to physical adsorption. The increase in Ea in the presence of cefuroxime axetil indicates physical or weak bonding between the molecules of the inhibitor and the mild steel surface. The standard free energy of adsorption ∆Gads can be calculated using the relation
log C = log θ /(1- θ) – log B
where, log B = -1.74 – (ΔG /2.303 RT)
θ = Surface coverage, C = Concentration, R = Gas Constant and T = Temperature
The negative values of ∆Gads (Table 4) ensure the spontaneity of the adsorption process and the stability of the adsorbed layer. The value of ΔG, indicates that the inhibitor function by physically adsorbing on the surface of the mild steel. Generally values of ΔG up to -20 kJ mol-1 are consistent with electrostatic interaction between charged molecules and charged metal (which indicates physisorption) while those more negative than – 40 kJ mol-1 involves charge sharing or transfer from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (which indicates chemisorptions) 22.
Synergistic effects of halide ions
Synergism between the organic inhibitor and halide ions on metal corrosion in acidic solution has been researched by many authors 23. Many studies indicate that nitrogen and sulphur containing organic compounds have been found to behave better for the steel corrosion in hydrochloric acid than in sulphuric acid. The possible reason is that there is a synergistic inhibition between chloride ion and such organic compounds 24. In the present study, an attempt has been made to study the influence of halide ions on the corrosion inhibition of cefuroxime axetil for mild steel in 1 M H2SO4, by weight loss measurements. It is clear from the Table 3 that addition of halide ions enhanced the inhibition efficiency of the inhibitor.
Table 3: Effect of anions on corrosion rate for 2mM of Cefuroxime Axetil in 1M H2SO4
|
Name of the anion |
Concentration of anion (mM) |
Weight loss (g) |
Corrosion rate (mmpy) |
Inhibition efficiency (%) |
|
Cl– |
2 |
0.0066 |
10.0452 |
96 |
|
I– |
2 |
0.0020 |
3.0440 |
99 |
Explanation for synergism
The compound contains nitrogen with unshared electron pair and p -electrons. In strongly acidic solution, they may be surface contains positive charge due to Ecorr – Eq=0 > 0 [where Eq=0=potential of zero charge]25. Thus, it is difficult for the positively charged inhibitor molecules to approach the positively charged steel surface due to electrostatic repulsion. Addition of halide ions causes their specific adsorption on the steel and causes the steel surface negatively charged. The protonated organic inhibitor is then adsorbed by coulombic attraction on the metal surface and therefore their inhibition efficiency is increased. The synergistic effect increases in the order Cl- <I-. This is because iodide ion is the most adsorbable of halide ions on steel.
Adsorption isotherm
Adsorption isotherms are very important in determining the mechanism of organic electrochemical reactions. The most frequently used isotherms are Langmuir, Temkin and Frumkin. The compounds follow Langmuir adsorption isotherm, which is given as
θ/(1 − θ ) =KC
Rearranging this equation
C / θ =1/ K + C
where θ is the surface coverage degree, K the equilibrium constant of the adsorption process and C is the inhibitor concentration. It was found that a plot of C/ θ vs. C is a straight line for cefuroxime axetil. Fig.3 depicts the graph of the Langmuir adsorption isotherm for the studied compound. As adsorption is of Langmuir character, the organic molecules are attached as a monolayer and through a physical mechanism.
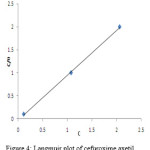 |
Figure 4: Langmuir plot of cefuroxime axetil Click here to View figure |
Table 4: Activation energy Ea and free energy of adsorption (ΔG ads) for the corrosion of mild steel in 1 M H2SO4 concentration of cefuroxime axetil.
|
S.No |
Concentration (mM) |
Ea (kJ/mol)
|
ΔG ads |
||
|
308 K |
313 K |
318 K |
|||
|
1 |
Blank |
4.874 |
– |
– |
– |
|
2 |
2 |
76.80 |
-15.2341 |
-14.3382 |
-13.3202 |
Polarization studies
The inhibition process of the cefuroxime axetil for the corrosion of mild steel in 1 M H2SO4 was analyzed by polarization experiments. Fig. 4 shows the Tafel anodic and cathodic polarization plots for the inhibitor cefuroxime axetil. Table 5 gives the values of electrochemical corrosion parameters. The lower corrosion current density (Icorr) values in the presence of inhibitors without causing significant changes in corrosion potential (Ecorr) and Tafel slopes ba and bc suggests that the compound is mixed type inhibitor and are adsorbed on the surface there by blocking the corrosion reaction.
Table 5: Potentiodynamic polarization parameter for mild steel in 1M H2SO4 with various concentration of Cefuroxime Axetil
|
Concentration of the Inhibtor(mM) |
Tafel slope |
Ecorr (mV/sec) |
Icorr (A/cm2) |
Corrosion rate (mmpy) |
I.E(%) |
|
|
ba(mV) |
bc(mV) |
|||||
|
Blank |
146.16 |
128.71 |
-442 |
107 |
4962.2 |
– |
|
0.001 |
126.75 |
103.68 |
-464 |
49 |
2260.8 |
54 |
|
0.1 |
138.0 |
107.28 |
-457 |
43 |
1996.1 |
60 |
|
1 |
90.85 |
86.558 |
-481 |
7.8 |
363.05 |
93 |
 |
Figure 5: Potentiodynamic polarization curves for mild steel in 1 M H2SO4 in the absence and presence of selected concentrations of cefuroxime axetil Click here to View figure |
Electrochemical Impedance Spectroscopy studies
The anticorrosive performance of the inhibitor was also studied by electrochemical impedance spectra at 30±1°C for various concentrations of the inhibitor in 1 M H2SO4. The data obtained are given in Table 6. The Rct is increased in the presence of cefuroxime axetil. Maximum increase was observed for at 4 mM concentration. The double layer capacitance Cdl decreased, as concentration is increased. This decrease may be due to the adsorption of the compounds on the metal surface leading to a film formation. The corresponding Nyquist diagram for cefuroxime axetil is shown in Fig. 5. As can be noticed, the impedance diagrams are perfect semicircles, indicating a charge transfer process mainly controlling the corrosion of the steel.
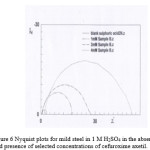 |
Figure 6 Nyquist plots for mild steel in 1 M H2SO4 in the absence and presence of selected concentrations of cefuroxime axetil. Click here to View figure |
Table 6: Impedance parameter for mild steel in 1M H2SO4 with various concentration of cefuroxime axetil
|
Concentration(mM) |
Rct (ohm.cm2)
|
Cdl x I0-5(Farads) |
Inhibition Efficiency(%) |
|
Blank |
1.20 |
40.90 |
– |
|
1.00 |
15.86 |
23.78 |
92 |
|
2.00 |
28.76 |
24.93 |
96 |
|
4.00 |
49.13 |
17.19 |
98 |
Conclusion
Inhibtion efficiencies of the inhibitor increase with increase in inhibitor concentrations. The optimum inhibitor efficiency of cefuroxime axetil has been achieved even at very low concentration of the inhibitor (2mM).
Temperature has significant in corrosion rate and inhibitor efficiency. Efficiency decreases with increase in temperature shows the desorption of the inhibitor at higher temperature from the metal surface.
The inhibition of corrosion by cefuroxime axetil is due to physisorption of the inhibitor on the metal surface. The greater Ea value in the presence of inhibitor and the less negative ∆Gads values support this point.
The variation of Tafel constants ba and bc and Ecorr values with increase in the concentration of inhibitor suggests that this compound acts as mixed type inhibitor.
The adsorption of inhibitor on the metal surface follows Langmuir isotherm.
References
- Zucchi F, Grassi V, Monticelli C and Trabanelli G., Corros Sci., 48, 522(2006).
- Bekkouch K, Aouniti A, Hammouti B and Kertit S., Corros Sci., 45, 1619-1630(2003).
- Refaey S. A. M, Taha F and Abd.El-Malak A M., Appl Surf Sci., 236, 175(2004).
- Mathiyarasu J, Palaniswamy, Subramanian P and Rengaawamy., Bull Electrochem., 14, 4-5,145 (1998).
- Rengamani S, Vasudevan T and Iyer S.V.K., Indian J.Technol., 31,519 (1993).
- Moretti G, Quartorone A, Tassan A and Zingates A., Werkst Karros., 45-12, 5 (1994).
- Hoar J.P and Khera R.P, Proc. 1st Eilr symp., On corros. Ing., Ferrara, Italy (1960).
- Moretti G, Quartorone A, Tassan A and Zingates A , Proc.of the Symp., Org compounds containing N,O,S and Se.,81 (1992).
- Eldakar N and Nobe.K., Corrosion Sci., 32.6,238 (1976).
- Mernari B, Attari H.E.L, Maisnel M, Bentiss F and Lagrence M., Corros.Sci., 40-2,391 (1998).
- Quraishi M.A, Muralidharan S and Iyer S.V.K., Anti corrosion methods and material.s,47,6, 354 (2000).
- Quraishi M.A, Mohn qusion, Ansari, Shanim Ahmed and Venkatachri G., Bull. Electrochem., 410, 302 (1998).
- Quraishi M.A, Rawat J,Ajmal M., Journal of applied electrochemistry., 30,6 ,745 -751(2000).
- Elkadi L,Mernari B, Traisnel M, Bentiss F and Lagrence., Corros Science., 42,703 (2000).
- Ajmal M, Mideen A.S and Quraishi M.A., Corros.Sci., 36-1,79 (1994).
- Muralidharan S, Chandrasekar R and Iyer S.V.K Proc., Indian Acad. Sci., (Chem. Sci)., 112-2,127 (2000).
- Quraishi M.A, Ansari. F.A, Jamal D., Materials chemistry and physics., 77 ,3, 687-690 (2003).
- Subramaniam G, Balasubramanian K and Sridhar P, proceedings of the 7th Eur.Smp.On corros.Inh. App.Driv.Ferrara,N.S.sez.9, 391 (1990).
- Schorr M.and Yahalom J., Corros Sci.,12,867-868(1972).
- Szauer T, Brandt., Electrochim. Acta., 26 (1981).
- Foroulis Z.A., Proceedings of the seventh European symposium corrosion inhibitors., Ferrara,149(1990).
- El-Etre A Y, J Colloid Interface Sci., 2007, 314(2), 578 – 583.
- Khamis E, El-Ashry E.S.H, Ibrahim A.K., Br. Corros. J., 35, 150- 154(2000).
- Xueming Li, Libin Tang, Lin Li, Guannan Mu, Guangheng Liu, Corros. Sci., 48 308-321(2006).
- Ye K.M., Mater. Protec., (Chinese) 23, 37-42(1990).

This work is licensed under a Creative Commons Attribution 4.0 International License.









