Comparative Study of Adsorption Isotherms two Non-Steroidal Anti-Inflammatory Eye Drops, Indomethacin and Diclofenac on Carbon Nanotube
Mehdi Vadi
Department of Chemistry, Fasa Branch, Islamic Azad University, Fasa, Fars, Iran.
We have studied the interaction of indomethacin and diclofenac solutions, on multi-walled carbon nanotube. After investigated comparative study and assigned to either indomethacin or diclofenac adsorption isotherms. The adsorption equilibrium isotherms were fitted by Freundlich, Langmuir, and Temkin models. It was found that the Freundlich model described the adsorption process better than other two isotherm models. The amount of NSAIDs (indomethacin and diclofenac) adsorbed on carbon nanotube surface increased with the increase of the initial NSAIDs concentration. The results show that diclofenac has most amount adsorption rate of MWCNT.
KEYWORDS:Adsorption; Isotherms; Non-steroidal; Carbon nanotube
Download this article as:| Copy the following to cite this article: Vadi M. Comparative Study of Adsorption Isotherms two Non-Steroidal Anti-Inflammatory Eye Drops, Indomethacin and Diclofenac on Carbon Nanotube. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Vadi M. Comparative Study of Adsorption Isotherms two Non-Steroidal Anti-Inflammatory Eye Drops, Indomethacin and Diclofenac on Carbon Nanotube. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23941 |
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their effect by virtue of cyclo-oxygenase inhibition in the arachidonic acid cascade (1, 2). Of these compounds, diclofenac sodium is also known to interfere with the lipoxegenase pathway (3). Moreover, due to their additional analgesic effect, (4, 5, and 6) these drugs are widely used to manage posttraumatic or postoperative pain and pain associated with the musculoskeletal disorders. Since NSAIDs inhibit prostaglandin synthesis, they are equally
recommended to treat allergic ocular disorders (7, 8). Several ophthalmic formulations of NSAIDs are marketed for topical use to manage pain and inflammation. Corticosteroids, Which exert a profound anti-inflammatory effect; have no direct influence on pain? In contrast to the corticosteroids, NSAIDs do not cause raised ocular tension, secondary cataract formation or reactivation of herpes simplex virus infection upon application to the eye. NSAIDs are especially prescribed to manage pain after refractive laser surgery (9, 10). They are helpful in maintaining my diesis during cataract surgery (11). Chronic oral ingestion of NSAIDs for analgesic, anti-inflammatory, and antithrombotic indications is associated with upper gastrointestinal tract lesions ranging from petechiae and superficial erosions to chronic peptic ulcers, oesophagitis, and gut ulcers which may perforate or cause gastrointestinal bleeding (12). The NSAIDs have been shown to inhibit the proliferation of mucosal cells that normally leads to healing of gastric and duodenal ulcers (13, 14). Although diclofenac does not retard the healing of experimental epithelial erosions (15), nothing is known about the effect of NSAIDs on the normal corneal epithelium. We investigated the effect of NSAID eye drops on the carbon nanotube. Carbon nanotubes (CNTs) can be described as rolled hexagonal carbon networks that are capped by half fullerene molecules. There are three main types of carbon tubes: single-walled (SWNTs), double-walled (DWNTs) and multi-walled (MWNTs). CNTs can be synthesized using the arc-discharge method (16) catalytic chemical vapor deposition (CVD) (17), and laser ablation (18). The dimensions of these tubular structures range from 0.4 to 2 nm in diameter for SWNTs and from 2 to 100 nm for MWNTs. Both types have length typically ranging from 1 to 50 μm. The diameters of DWNTs are typically from 1 to 3.5 nm and they are from several micrometers to tens of micrometers in length (19). The possibility to combine the remarkable specificity and parallel processing of biomolecules with the hollowed cavity, size and electrical properties of carbon nanotubes has attracted considerable attention for several types of applications, ranging from the creation of new types of biosensors (20) to the fabrication of drug or vaccine delivery devices
It has been shown (21) that peptide–carbon nanotube complexes enhance the immune (antibody) response against the peptides with no detectable cross-reactivity to the carbon nanotubes (i.e. carbon nanotubes are not intrinsically immunogenic). Functionalized carbon nanotubes have been shown to cross cell membranes and to accumulate in the cytoplasm without being toxic for the cell (22).
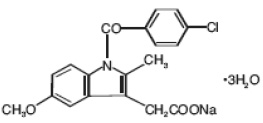
Indomethacin Formula
Indomethacin for injection is designated chemically as 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid, sodium salt, trihydrate. Its molecular weight is 433.82. Its empirical formula is C19H15ClNNaO4•3H2O and its structural formula is:
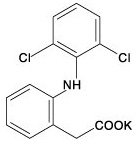
Diclofenac Formula
Diclofenac Potassium, USP is a benzene acetic acid derivative, designated chemically as 2-[(2, 6-dichlorophenyl) amino] benzene acetic acid, mono potassium salt. The molecular weight is 334.25. Its molecular formula is C14H10Cl2NKO2. The structural formula is:
Adsorption experiments
First solution concentration of 100 mg / L of sample prepared and dilution of the solution, solution concentrations (5, 10, 20 and 30) mg / L were prepared. Each tube containing 0.01 g CNTs was filled with 10 mL indomethacin and diclofenac solution of different concentrations. All tubes were immediately sealed with PTFE-lined caps and were then mechanically shaken for 24 h in a thermo stated rotary shaker at temperature of 295 ± 1 K, were adjusted. After equilibration, all tubes were placed vertically for 4 h at the same temperature to ensure complete sedimentation of CNTs from the bulk solutions. By using on spectrophotometer tool adsorption rate, gained for NSAIDs.
Adsorption isotherms
Equilibrium study on adsorption provides information on the capacity of the adsorbent. An adsorption isotherm is characterized by certain constant values, which express the surface properties and affinity of the adsorbent and can also be used to compare the adsorptive capacities of the adsorbent for different pollutants. Equilibrium data can be analyzed using commonly known adsorption systems. Several mathematical models can be used to describe experimental data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment.
Langmuir model: The Langmuir adsorption model [28] is the most common model used to quantify the amount of adsorbate on an adsorbent as a function of partial pressure or concentration at a given temperature. This equation expressed by relation.

In this equation, qe (mg.g-1) is the solution was adsorbed the surface and qm is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and Ce (mg.L-1) is solution in equilibrium state.
Freundlich model: The Freundlich equation or Freundlich adsorption isotherm is an adsorption isotherm, which is a curve relating the concentration of a solute on the surface of an adsorbent, to the concentration of the solute in the liquid with which it is in contact. In 1909, Freundlich gave an empirical expression representing the isothermal variation of Adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with pressure. This equation is known as Freundlich Adsorption Isotherm or Freundlich Adsorption equation. This model is specified with equation.
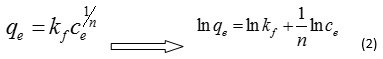
In this equation, qe (mg.g-1) is amount of absorbed material in absorbent surface, K, n in arrangement are adsorption capacity and adsorption intensification.
Temkin model: The Temkin model is linearly represented as equation (3) and generally applied in the form:
![]()
Where A and B are the Temkin isotherm constant (L/g) and heat of sorption (J/mol) respectively. R is the gas constant (J/mol/k), b is the Temkin isotherm constant linked to the energy parameter, B, as shown on equation (4):
b = RT/B (4)
T is the absolute temperature in Kelvin.
Results and discussion
Adsorption isotherms
The Langmuir, Freundlich and Temkin isotherms of the adsorption process of NSAIDs on CNT are shown in figures 1 to 3 and calculated parameters of these models are shown in Table 1. It was observed that the experimental data were well represented by Langmuir Freundlich and Temkin models.
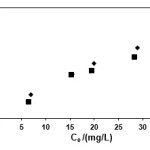 |
Figure 1: Langmuir isotherm of indomethacin and diclofenac on CNT |
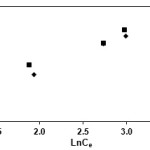 |
Figure 2: Freundlich isotherm of indomethacin and diclofenac on CNT |
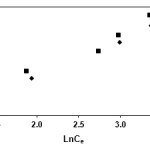 |
Figure 3: Temkin isotherm of indomethacin and diclofenac on CNT |
Table 1: Parameters of Langmuir, Freundlich and Temkin isotherms of the indomethacin and diclofenac on CNT
|
|
Langmuir |
Freundlich |
Temkin |
|||||||
|
b |
q |
R2 |
n |
K(L.g-1) |
R2 |
A(L.mg-1) |
B |
b(J.mol-1) |
R2 |
|
|
Indomethacin |
0.0673 |
7.006 |
0.989 |
2.105 |
1.148 |
0.990 |
0.118 |
2.758 |
11.452 |
0.982 |
|
Diclofenac |
0.0698 |
8.235 |
0.953 |
2.222 |
1.473 |
0.989 |
0.089 |
2.457 |
11.014 |
0.900 |
Conclusions
We have studied the interaction of indomethacin and diclofenac with as-prepared and purified multi-walled carbon nanotubes (MWCNTs). Optical spectroscopic data Uv/Vis showed that purified carbon nanotubes interact with indomethacin and diclofenac. These experimental results are confirmed by first principles calculations that predict a very weak adsorption process through p–p interaction instead of through the free electron pair of the N and Cl .Spectroscopic studies showed that the interaction of indomethacin and diclofenac with carbon nanotubes is weak, in agreement with theoretical predictions based on ab initio calculations that indicate that for both metallic and semiconducting nanotubes the interaction occurs via p–p orbital interaction between the indomethacin and diclofenac aromatic rings and the C-ring of the MWCNTs, instead of through the free electron pair of the N and Cl. In this research, adsorption some of Non-steroidal anti-inflammatory drugs eye (indomethacin and diclofenac) on multiwall carbon nanotube from aqueous solution were studied. The results show that the amount of indomethacin and diclofenac adsorbed on carbon nanotube surface increased with the increase of the initial NSAIDs concentration. The results show that diclofenac has most amount adsorption rate of MWCNT because it can be attributed to lower the barrier of space in this material. The experimental results show that it was found that the Freundlich model described the adsorption process better than other two isotherm models.
References
- Ku u. C., Lee W., Kothari h. V., Scholerd. W.: Effect of diclofenac sodium on the arachidonic acid cascade. Am J Med., 1986,80: 18-23.
- Terasawam. Imayoshit., Iwahisay., Maruyamay.: Anti-inflammatory effect of topically Applied pranoprofen-gel. Nippon-Yakurigaku- Zasshi, 1985, 85: 283-296.
- Appiotti A., Gualdil. Albertim. Gualdim. Comparative study of the analgesic efficacy Of flurbiprofen and diclofenac in patients following excimer laser photorefractive keratectomy. Clin-Ther., 1998, 20: 913-920.
- Chen X., Gallar J., Belmonte C.: Reduction by anti-inflammatory drugs of the response Of corneal sensory nerve fibers to chemical irritation. Invest Ophthalmol Vis Sci., 1997, 38: 1944-1953
- Terasawa M., Yakushiji T., Iwahisa Y., Imayoshi T., Maruyama Y.: Analgesic effect Of topically applied pranoprofen-gel. Nippon-Yakurigaku-Zasshi, 1985, 86: 433-440.
- Lee W.C., Morgan D.W., Marriott J.F.: Are prostaglandins major mediators in perennial allergic rhinitis? Rhinology, 1996, 34: 130-135.
- Van husen H.: Lokale Behandlung mit Diclofenac- Na-Augentropfen bei Erkrankungen der vorderen Augenabschnitte. Klin. Monatsbl. Augenheilkd., 1986, 615-619.
- Arshinoff S., D’ Addario D., Sadler C., Bilotha R., Johnson T.N.: Use of topical Nonsteroidal anti-inflammatory drugs in excimer laser photorefractive keratectomy. Cataract Refract. Surg., 1994, 20: 216-222.
- Assouline M., Renard G., Arne J. L., David T., Lasolis C., Malecaze F., Pouliquen
- Y. J.: A prospective randomized trial of topical soluble 0.1% indomethacin versus 0.1% diclofenac versus placebo for the control of pain following excimer laser photorefractive keratectomy. Ophthalmic Surg. Lasers, 1998, 29: 365-374.
- Kotharih. V., Lee W. H., Kue. C.: An alternate mechanism for regulation of leukotrieneProduction in leukocytes: studies with an anti-inflammatory drug, sodium diclofenac. Biochim-Biophys-Acta, 1987, 921: 502-511.
- Herschowitzb .I: Nonsteroidal anti-inflammatory drugs and the gut. South. Med. J., 1996,89: 259-263.
- Levis., Goodladr .A., Lee C.Y., Stampg., Walportm.J., Wrightn.A. HodgsonH.J.: Inhibitory effect of non-steroidal anti-inflammatory drugs on mucosal cell proliferation associated with gastric ulcer healing. Lancet, 1990, 336: 840-843.
- Levis., Goodladr.A., Lee C.Y., Stamp G., Walport M.J., Wrightn.A., Hodgson H.J.: Non-steroidal anti-inflammatory drugs inhibit the process of mucosal cell proliferation associated with duodenal ulcer healing. Digestion, 1992, 53: 129-133.
- Loyan., Bassages., Vyass., Del-Cerrom., Parks.B., Aquavellaj.V.: Topical diclofenac Following excimer laser: effect on corneal sensitivity and wound healing in rabbits.J. Refract. Corneal Surg., 1994, 10: 423-427.
- Maudgalp.C., Cornelisp., Missottenl . Effects of commercial ophthalmic drugs on rabbit Corneal epithelium. Von Graefes Klin Ophthalmol., 1989, 216: 191-203.
- Ajayan et al., 1993 P.M. Ajayan, J.M. Lambert, P. Bernier, L. Barbedette, C. Colliex and J.M. Planeix, Growth morphologies during cobalt-catalyzed single-shell carbon nanotube synthesis, Chem. Phys. Lett. 215 (1993), pp. 509–517.
- Dai et al., 1996 H. Dai, A.G. Rinzler, P. Nicolaev, A. Thess, D.T. Colbert and R.E. Smalley, Single-wall nanotubes produced by metal-catalyzed disproportionation of carbon monoxide, Chem. Phys. Lett. 260 (1996), pp. 471–475.
- Diaz et al., 2000 A. Diaz, A.C. Willis and R.B. Sim, Expression of the proteinase specialized in bone resorption, cathepsin K, in granulomatous inflammation, Mol. Med. 6 (2000), pp.648–654.
- Flahaut et al., 2003 E. Flahaut, R. Bacsa, A. Peigney and C. Laurent, Gram-scale CCVD synthesis of double-walled carbon nanotubes, Chem. Commun. 12 (2003), pp. 1442–1443.
- Briand and M. Prato, Amino acid functionalization of water soluble carbon nanotubes, Chem. Commun. 24 (2002), pp. 3050–3051.
- Guo et al., 1995 T.M. Guo, P. Nikolaev, A. Thess, D.T. Colbert and R.E. Smalley, Catalytic growth of single-walled nanotubes by laser vaporization, Chem. Phys. Lett. 243 (1995), pp. 49–54.
- Pantarotto et al., 2004 D. Pantarotto, J.P. Briand, M. Prato and A. Bianco, Translocation of bioactive peptide across the cell membranes by carbon nanotubes, Chem. Commun. 1 (2004),pp. 16–17.

This work is licensed under a Creative Commons Attribution 4.0 International License.









