An Efficient Synthesis of Schiff Bases Containing Benzimidazole Moiety Catalyzed by Bismuth Trichloride Under Microwave Irradiation
A. Thirupathaiah1* and D. Dasharatham2
1Kishan pura, Hanmakonda, Warangal – 506 001 (India).
2Department of Chemistry,Kakatiya University, Warangal - 506 009 (India).
A simple and efficient method has been developed for the synthesis of some novel Schiff bases via the reaction of aromatic aldehydes with 2-aminobenzimidazole by using catalytic amount of BiCl3 in an organic solvent under microwave irradiation. Some advantages of this protocol are its very good yields, use of available catalysts, simple workup procedure, and short reaction times.
KEYWORDS:Aldehyde; 2-aminobenzimidazole; catalyst; Schiff bases; BiCl3; microwave irradiation
Download this article as:| Copy the following to cite this article: Thirupathaiah A, Dasharatham D. An Efficient Synthesis of Schiff Bases Containing Benzimidazole Moiety Catalyzed by Bismuth Trichloride Under Microwave Irradiation. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Thirupathaiah A, Dasharatham D. An Efficient Synthesis of Schiff Bases Containing Benzimidazole Moiety Catalyzed by Bismuth Trichloride Under Microwave Irradiation. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=24062 |
Compounds containing a benzimidazole moiety attached to a heterocyclic system are important chemical classes as a result of their significant biological activities against several viruses such as HIV, herpes (HSV-1), influenza, and Epstein-Barr [1−3]. Moreover, benzimidazole derivatives have been studied as anticancer and antiproliferative chemicals [4,5]. Schiff bases derived from aromatic amines and aromatic aldehydes are also a very important class of organic compounds because of their applications in many fields including biological [3,6−11], inorganic, [12−16] and analytical chemistry [17−21]. The hybrid molecules composed of the combination of part of a heterocyclic ring, like benzimidazole, and part of the Schiff base may exert potential biological activities. Several synthetic methods have been reported for the synthesis of Schiff bases. However, most of them have limitations including long reaction times, need for a special catalyst, low yields, and extensive recrystallization [22−25]. Therefore, the pursuance of more convenient and practical synthetic methods for preparation of these compounds still remains an active research area. Recently, the use of several catalysts, like inorganic salts [26] and zeolites, [27, 28] in organic synthesis has attracted considerable attention. They have many advantages such as their handling, low cost, and being environmentally safe. Because of the non-toxic nature and mild Lewis acid activity of bismuth (III) chloride, we and others have been using this as an efficient green catalyst for the production of vital organic compounds [29 – 37].
In this communication, described herein is the synthesis of synthesis of Schiff bases [38], we studied a simple and efficient synthetic method for the preparation of Schiff bases containing benzimidazole moiety employing bismuth (III) chloride as a mild, green, and efficient catalyst under microwave irradiation (Scheme 1).
All chemicals used were purchased from Merck or Fluka. Melting points were determined using an electrothermal digital apparatus and are uncorrected. FT-IR spectra were registered on a Bruker IFS 55 Equinox FTIR spectrophotometer as KBr discs. NMR spectra were recorded on a Bruker (300 MHz) spectrometer. Chemical shifts (ppm) were referenced to the internal standard tetramethylsilane (TMS). Reactions were monitored by thin layer chromatography (TLC).
Initially we sought a mild and convenient method for the synthesis of benzimidazole Schiff bases at room temperature. For optimization of the amount of catalyst, the reaction of 2-aminobenzimidazole with 4-nitrobenzaldehyde at ambient temperature was carried out as a model reaction. The use of 5 mol% of BiCl3 in methanol for 30 minutes afforded the corresponding Schiff base in 77% yield.
The optimization of the other reaction conditions was undertaken to increase the yield employing the BiCl3 (5 mol%) was added and the reaction mixture was taken into a pyrex cylinder and irradiated in a microwave oven (450W), at the end of irradiation (10 – 25 s). The results are summarized in Table 1. The yield of reaction in the presence of 5 mol% of BiCl3 under microwave irradiation was increased up to 95%. To study the development of this method, the optimized procedure was extended for preparation of other Schiff bases. The reaction was carried out under microwave irradiation by taking a 1:1 mol ratio mixture of 2-aminobenzimidazole 1 and the corresponding aromatic aldehyde 2 in the presence of 5 mol% of BiCl3 in methanol to give Schiff bases 3a-l (Scheme 1).
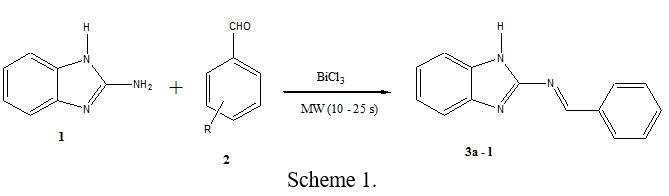
Table 1: Schiff bases derived from reaction of 2-aminobenzimidazole with aromatic aldehydes in the presence of 5 mol% of BiCl3 under microwave irradiation.
| Compound | R | Time (sec) | Mp (°C) | Yield (%)b |
| 3a | H | 20 | 149 – 152 (152 – 154)a | 94 |
| 3b | 2-Cl | 18 | 205 – 207 (212 – 217) | 78 |
| 3c | 3-Cl | 17 | 212 (200 – 202) | 90 |
| 3d | 4-Cl | 20 | 230 – 231 (179 – 181) | 87 |
| 3e | 4-Br | 20 | 245 – 2246 (256 –257) | 88 |
| 3f | 3-NO2 | 20 | 191 – 193 | 80 |
| 3g | 4-NO2 | 16 | 264 (266 – 268) | 95 |
| 3h | 4-Me | 22 | 212 – 213 (226 – 228) | 89 |
| 3i | 4-OMe | 25 | 222 – 223 (220 – 222) | 82 |
| 3j | 2-OH | 16 | 225 – 227 (217 – 218) | 90 |
| 3k | 3-CHO | 25 | 257 – 261 | 78 |
| 3l | 4-CHO | 20 | > 300 | 93 |
aMelting points of the 3a – j reported in the literature[30−34]
bIsolated yields
The yields of reactions using this practical procedure for the preparation of various Schiff bases in comparison with the previously reported methods [39−43] are quite fair and the reaction times are very short. Aliphatic aldehydes or ketones such as formaldehyde, acetaldehyde, and acetophenone were also examined under the same conditions, but the corresponding products were isolated in trace amounts. The structure of products was characterized by the spectroscopic data. The 1H- and 13C-NMR spectra of all synthesized Schiff bases are consistent with their structures. The 1H-NMR spectra of these compounds are simple and consist of the aromatic protons signals and 2 singlet signals related to the resonance of the C-H and N-H proton, which appeared at 9.38 – 9.78 and 12.62–12.89 ppm, respectively [44]. The aromatic protons resonate as a multiple signal at 6.88–8.88 ppm depending on the Ar group.
In summary, the present method employing BiCl3 is mild, efficient, and environment benign green protocol for the synthesis of Schiff bases containing benzimidazole moiety by reaction of 2-aminobenzimidazole and aromatic aldehydes. The products are obtained in high yields and the reaction time is short. The present protocol employs a catalyst that is inexpensive and a well-known non-toxic inorganic salt. Furthermore, the process is carried out with operational simplicity and simple work-up procedures. These features place this protocol at an advantage to the existing processes.
Acknowledgement
The authors are thankful to Dr. K. Raji Reddy, Sr. Scientist, IICT, Hyderabad for his constant encouragement and suggestions to complete this work.
References
- I. Tamm, P. B. Sehgal, Adv. Virus. Res. 22 (1978) 186.
- I. Tamm, Science 120 (1954) 847.
- M. M. Ramla, A. M. Omar, H. Tokudo, I. H. El-Diwoni, Bioorg. Med. Chem. 15 (2007) 6489.
- Z. Kazimierzcuk, D. Shugar, Nucleosides Nucleotides 8 (1989) 1379.
- R. Ottana, S. Carotti, R. Maccari, et al. Bioorg. Med. Chem. Lett. 15 (2005) 3930.
- G. G. Mohamed, M. M. Omar, A. M. Hindy, Turk. J. Chem. 30 (2006) 361.
- I. Yildiz-Oren, I. Yalcin, E. Aki-Sener, N. Ucarturk, Eur. J. Med. Chem. 39(2004) 291.
- C.S. Ra, B.Y. G .Jung, Park, Heterocycles 62 (2004) 793.
- A.E. Taggi, A. M. Hafez, H. Wack, B. Young, D. Ferraris, T. Lectak, J. Am. Chem. Soc. 124 (2002) 6626.
- J. M. Hearn, M. H. Cynamon, J. Antimicrob. Chemother. 53 (2004) 185.
- W. P. Nawrocka, B. Sztuba, A. Drys, J. Wietrzyk, J. Kosendiak, A.Opolski, Pol. J. Chem. 80 (2006) 279.
- E. Canpolat, M. Kaya, Turk. J. Chem. 29 (2005) 409.
- R. Ramesh. M. Sivagamasundari, Syn. Reac. Inorg. Met-org. Chem. 33 (2003) 899.
- Liu, J.; Wu, B. Zhang, B.; Liu, Y. Turk. J. Chem. 2006, 30, 41-48.
- G. G. Mohamed, Z. H. Abd El-Wahab, J. Therm. Anal. Cal. 73 (2003) 347.
- A. Albinati, C. Arz, H. Berger, P. S. Pregosin, Inorg. Chim. Acta 190 (1991) 119.
- P. L. Croot, M. Johansson, Electroanalysis 12 (2000) 565.
- M. A. Abdul-Munem Ali, A. M. A. H. Al-Haideri, M. T. S. Al-Mehdawy, Turk. J. Chem. 27 (2003) 259.
- M. R. Ganjali, P. Norouzi, N. Hatambeygi, M. Salavati-Niasari, J. Braz. Chem. 17 (2006) 859.
- M. Salavati-Niasari, J. Chemistry Letters 34 (2005) 1444.
- F. Shemirani, A. A. Mirroshandel, M. S. Niasari, R. R. Kozani, J. Anal. Chem. 59 (2004) 228.
- J. E. Dos Santos, E. R. Dockal, E. T. G. Cavalheiro, Carbohydr. Polym. 60 (2005) 277.
- H. Naeimi, H. Sharghi, F. Salimi, Kh. Rabiei, Heteroat. Chem 19 (2008) 43.
- J. Parekh, P. Inamdhar, R. Nair, S. Baluja, S. Chanda, J. Serb. Chem. Soc. 70 (2005) 1155.
- H. J. Yang, W. H. Sun, Z. L. Li, Z. Ma, Chin. Chem. Lett. 13 (2002) 3.
- A. Mobinikhaledi, P. J. Steel, Syn. Reac. Inorg. Met-org. Chem. 39(2009) 133.
- A. Mobinikhaledi, N. Foroughifar, M. Zendehdel, M. Jabbarpour, Syn. Reac. Inorg. Met-org. Chem. 38 (2008) 390.
- A. Mobinikhaledi, N. Foroughifar, N. Bassaki, Turk. J. Chem. 33 (2009) 555.
- B. Baruah, A. Baruah, D. Prajapati, J.S. Sandhu, Tetrahedron Lett. 38 (1997) 1449.
- H.N. Borah, D. Prajapati, J.S. Sandhu, A.C. Ghosh, Tetrahedron Lett. 35 (1994) 3167.
- A. Boruah, B. Baruah,; Prajapati, D.; Sandhu, J.S. Synlett. 1997, 1251_1252.
- A.J. Thakur, A. Boruah, D. Prajapati, J.S Sandhu, Synth. Commun. 30 (2000) 2105.
- M. Wada, H. Ohki, K.Y. Akiba, Bull. Chem. Soc. Jpn. 63 (1990) 1738.
- H. Suzuki, T. Ikegami, Y. Matano, Synthesis. (1997) 249.
- S. Vidal, Synlett. (2001) 1194.
- S.K. De, R.A. Gibbs, Synthesis. (2005) 1231.
- K. Ramalinga, P. Vijayalakshmi, T.N.B. Kaimal, Synlett.(2001) 863.
- A. Mobinikhaledi, P. J. Steel, M. Palson, Syn. Reac. Inorg. Met-org. Chem. 39 (2009) 189.
- A. E. Abdel-Rahman, A. M. Mahmoud, G. M. El-Naggar, H. A. El-Sherief, Pharmazie 38 (1983) 589.
- A. Cuadro, J. Perez-Butragueno, M. Pastor-Maeso, et al. Farmaco 47 (1992) 477.
- A. Albinati, C. Arz, P. S. Pregosin, J. Organomet. Chem. 356 (1988) 367.
- M. Pedro, A. Enrique, L. Angeles, Heterocycles 37 (1994) 997.
- W. Nawrocka, B. Sztuba, M.W. Kowalska, et al. Farmaco 59 (2004) 83.
- General preparation of Schiff bases 3a-l: To a solution of 2-aminobenzimidazole (1 mmol) in methanol (5 mL) was added corresponding aromatic aldehyde (1 mmol). Then BiCl3 (5 mol%) was added and the reaction mixture was taken into a pyrex cylinder and irradiated in a microwave oven (450W) at the end of irradiation (10 – 25 s), After completion of the reaction, cold water (15 – 25 mL) was added to give the product. The solid product was filtered and washed with cold water and air dried.3a:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.72 (s, 1H, NH), 9.46 (s, 1H, CH) and 8.06 – 6.88 (m, 9H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 165.8, 156.1, 136.6, 135.5, 135.0, 133.2, 129.9, 129.5, 122.4, 119.2 and 111.7 ppm. FT-IR (KBr): 3375 (w), 3059 (m), 1621 (s), 1575 (s), 1450 (s), 1275 (m), 1213 (w), 740 (s) and 688 (m) cm−1.3b:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.86 (s, 1H, NH), 9.78 (s, 1H, CH), 8.28 (d, J = 7.7 Hz, 1H, HArom), 7.65 – 7.48 (m, 5H, HArom) and 7.21 (q, 2H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 161.0, 155.6, 136.6, 134.6, 132.2, 130.8, 128.8, 128.3, 122.7, 119.4 and 111.7 ppm. FT-IR (KBr): 3053 (m), 2986 (w), 1604 (s), 1520 (m), 1427 (s), 1273 (m), 1053 (m), 756 (s), 684 (w) and 451 (w) cm−1.3c:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.78 (s, 1H, NH), 9.46 (s, 1H, CH) and 8.10-7.20 (m, 8H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 164.3, 155.6, 137.6, 134.3, 132.7, 131.4, 129.1, 128.5, 122.6, 119.1 and 111.9 ppm. FT-IR (KBr): 3161 (w), 3067 (m), 2995 (w), 1606 (m), 1568 (m), 1423 (s), 1211 (w), 1076 (w), 840 (m), 740 (s) and 677 (m) cm−1.3d:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.86 (s, 1H, NH), 9.46 (s, 1H, CH) and 8.08 – 7.18 (m, 8H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 164.5, 155.8, 137.8, 134.4, 131.5, 129.7, 122.5, 118.6 and 112.5 ppm. FT-IR (KBr): 3375 (w), 3065 (m), 2993 (w), 1612 (m), 1570 (m), 1500 (m), 1429 (s), 1311 (w), 1234 (w), 1089 (m), 821 (m), 738 (s) and 505 (w) cm−1.3e:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.75 (s, 1H, NH), 9.44 (s, 1H, CH) and 7.99 – 7.18 (m, 8H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 164.7, 155.6, 134.7, 132.6, 131.7, 126.9, 122.5, 119.3 and 111.8 ppm. FT-IR (KBr): 3422 (w), 3063 (m), 2991 (w), 1610 (m), 1489 (m), 1429 (s), 1070 (m), 819 (m) and 738 (s) cm−1.3f:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.86 (s, 1H, NH), 9.61 (s, 1H, CH) and 8.88 – 7.21 (m, 8H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 163.6, 155.4, 148.7, 137.1, 136.1, 131.1, 127.0, 123.4, 122.7, 119.7 and 111.4 ppm. FT-IR (KBr): 3346 (m), 3088 (m), 1608 (m), 1529 (s), 1431 (m), 1350 (s), 1278 (w), 1085 (w), 742 (s) and 665 (w) cm−1. 3g: Brown solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.89 (s, 1H, NH), 9.58 (s, 1H, CH), 8.37-8.16 (m, 4H, HArom) and 7.60 – 7.20 (m, 4H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 163.6, 155.2, 149.8, 141.0, 130.8, 124.6, 122.8, 119.3 and 111.8 ppm. FT-IR (KBr): 3167(m), 3080 (m), 1614 (w), 1591 (m), 1514 (s), 1425 (m), 1342 (s), 1228 (w), 1109 (w), 833 (m) 763 (s), 680 (w) and 441 (w) cm−1. 3h: Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.66 (s, 1H, NH), 9.41 (s, 1H, CH), 7.94-7.16 (m, 8H, HArom) and 3.38 (s, 3H, Me) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 165.6, 156.3, 143.6, 133.0, 130.2, 130.0, 122.3, 119.0, 111.5 and 35 (CH3) ppm. FT-IR (KBr): 3053 (m), 2862 (w), 1604 (s), 1525 (s), 1429 (s), 1280(s), 1174 (s), 1045 (m), 1001 (w), 815 (s), 742 (s), 503 (m) and 451 (w) cm−1.3i:Green solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.62 (s, 1H, NH), 9.38 (s, 1H, CH), 8.01 – 7.11 (m, 8H, HArom) and 3.83 (s, 3H, OMe) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 165.0, 163.4, 156.6, 132.0, 128.4, 122.2, 118.7, 115.0, 111.4 and 55.99 (OMe) ppm. FT-IR (KBr): 3002 (w), 2931 (w), 1601 (s), 1568 (s), 1525 (s), 1425 (s), 1307 (w), 1255 (s), 1165 (s), 1022 (m), 837 (m) and 744 (m) cm−1.3j: Yellow solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.78 (s, 1H, NH), 12.11 (s, 1H, OH), 9.66 (s, 1H, CH), 7.88 (d, J = 7.9 Hz, 1H, HArom), 7.58 – 7.47 (m, 3H, HArom), 7.21 (q, 2H, HArom) and 7.04 (t, J = 6.5 Hz, 2H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 165.9, 160.9, 154.3, 135.1, 132.7, 122.5, 120.1, 119.8, 119.0, 117.3 and 111.7 ppm. FT-IR (KBr): 3236 (w), 3047 (w), 1608 (s), 1570 (m), 1520 (w), 1431 (s), 1278 (s), 1192 (w), 1151 (w), 866 (w) and 746 (s) cm−1.3k:Yellow solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.80 (br, 2H, NH), 9.56 (s, 2H, CH), 8.57 (s, 1H, HArom), 8.13 – 8.36 (m, 3H, HArom) and 6.99 – 7.78 (m, 8H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 164.7, 155.8, 137.2, 136.4, 134.2, 133.5, 130.6, 122.6 and 111.7 ppm. FT-IR (KBr): 3367 (w), 3063 (w), 1616 (s), 1579 (s), 1516 (m), 1438 (s), 1275 (m), 1149 (m), 999 (w), 798 (w) 744 (s), 680 (m) and 432 (w) cm−1.3l: Dark yellow solid; 1H-NMR (300 MHz, DMSO-d6) δ : 12.82 (br, 2H, NH), 9.55 (s, 2H, CH), 8.25 (s, 4H, Hphenylene),7.54 (m, 4H, HArom) and 7.20 (m, 4H, HArom) ppm. 13C-NMR (75 MHz, DMSO-d6) δ : 164.7, 155.6, 140.4, 138.9, 130.4, 122.7 and 112.8 ppm. FT-IR (KBr): 3337 (m), 3051 (w), 1612 (s), 1568 (m), 1437 (s), 1309 (w), 1205 (s), 812 (m) and 746 (m) and 505 (m) cm−1.

This work is licensed under a Creative Commons Attribution 4.0 International License.









