Adsorption of Zinc Ion on Leonardite Prepared from Coal Waste
Suttasinee Katanyoo1 , Wimol Naksata1, Ponlayuth Sooksamiti2 , Sakdiphon Thiansem3 and Orn-anong Arquero1*
1Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand 50200.
2Department of Primary Industry and Mine Office Region 3, Chiang Mai, Thailand 50200.3 Department of Industrial Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand 50200
Naturally occurring leonardite, obtained from Mae Moh mine lignite, has been tested as a potential sorbent for Zn2+. The factors that affect to the sorption, such as pH, contact time and the adsorption isotherm were also investigated. The high performance of Zn2+ removal was demonstrated at pH values of 5-6. Batch experiment showed that the equilibrium was reached after 180 min. The sorption data were correlated with the Langmuir and Freundlich adsorption models. The average maximum adsorption capacity was found to be 0.141 mmol g-1. Moreover, the equilibrium parameter, KR indicated the favor ability of leonardite for adsorbed Zn2+.
KEYWORDS:wastewater; adsorption; zinc; leonardite; coal waste
Download this article as:| Copy the following to cite this article: Katanyoo S, Naksata W, Sooksamiti P, Thiansem S, Arquero O. Adsorption of Zinc Ion on Leonardite Prepared from Coal Waste. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Katanyoo S, Naksata W, Sooksamiti P, Thiansem S, Arquero O. Adsorption of Zinc Ion on Leonardite Prepared from Coal Waste. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23954 |
Introduction
Heavy metal like zinc (Zn) is often found in plating, galvanizing and roller coating operations [1]. Althoughzinc is essential minerals but too much is not beneficial. The increase of zinc concentrations above the consent limits is becoming an important subject to public health [2]. Adsorption techniques employing solid sorbents provide an attractive alternative for the treatment of wastewater, especially if the sorbent is inexpensive and does not require an additional pre-treatment step (such as activation) before its application.
Leonardite or oxihumolite originated on the surface of lignite deposits by post-sedimentary oxidation. The oxihumolites, similarly to the related organic deposits such as humalites, are exploited for an industrial preparation of humic acids as well as additives to agricultural fertilizers [3-5]. Humic materials are complex organic molecules that contain a wide variety of functional groups: carboxyl (COOH), hydroxyl (OH) and carbonyl (C=O) that can be involved in chemical binding. Because of these properties, leonardite tends to have a high cation exchange capacity [6, 7]. The low-cost and the availability of this material make it a promising candidate for pollution remediation in both soils and groundwater. Therefore, the adsorption of zinc on untreated leonardite from the Mae Moh lignite mine in Lampang province was studied. The adsorption capacities can be estimated from the parameters of the adsorption isotherms, and factors influencing the adsorption are investigated.
Materials and methods
The sorbent ¾leonardite¾obtained from the Mae Moh lignite mine in Lampang province, Thailand, was used without any additional pre-treatment except of grinding and size classification by sieving (80 mesh). The surface area determined by the Brunauer-Emmett-Teller (BET) method using a Quantachrome Autosorb automate with nitrogen gas (version 2.46), after drying at 110°C for 12 hours was in the range 18.94-26.29 m2 g-1, and the average cation exchange capacity (CEC) measured to evaluate the adsorption capacity by the ammonium acetate method was found to be 61.95 cmol kg-1.
All reagents used are AR grade and heavy metal stock solutions were prepared with concentrations of up to 1000 mg/L of zinc nitrate salt in deionized water. The stock standard solution of zinc ion at concentration of 1000 mg/L was obtained from Fluka, Buches, Switzerland. Working standard solutions were obtained by appropriate dilution of the stock standard solution. Solutions of nitric acid (0.5 M) and sodium hydroxide (0.5 M) were used for pH adjustment.
Determination of zinc ion in the initial and remaining solutions was carried out by atomic absorption spectrophotometer, series AA-275, Varian Company, Australia.
Influence of pH on Zn2+ adsorption
The effect of pH on the adsorption of Zn2+ by leonardite was studied with aqueous solutions of zinc ion at 5 and 20 mg/L concentrations. The initial pH was adjusted to pH 2, 3, 4, 5 and 6 with nitric acid (0.5 M) or sodium hydroxide (0.5 M) solutions. Then, 0.4 g of the leonardite was added to flasks containing 50 mL of the zinc solutions. The flasks were maintained under continuous agitation for 24 h, at room temperature and 130 rpm. Then, the mixture was centrifuged and filtered, and the remaining zinc ion concentrations were determined.
A blank was prepared by adding 0.4 g of leonardite to 50 mL of DI water to verify whether the leonardite transferred zinc ion to water. Control test solutions (10 mL) were filtered and analyzed after pH adjustment to ensure that no precipitates formed prior to contact with the sorbent.
Effect of contact time
Batch experiments were conducted to determine the time needed to reach equilibrium at the optimum pH. 0.4 g leonardite was added to flasks containing 50 mL of 5 and 20 mg/L of Zn2+ aqueous solutions. The experimental conditions used were the same as those mentioned above. Samples were collected at various periods of time (10, 20, 30, 40, 50, 60, 120, 180, 240, 300 and 360 min), each mixture was centrifuged and filtered, and the remaining metal ion concentrations were determined.
Adsorption Isotherm
To test the adsorption capacity of leonardite, the experiments were carried out with aqueous solutions of Zn2+ at initial concentrations from 5 to 100 mg/L, with 0.4 g leonardite dose. The flasks were kept in the same conditions as described above until equilibrium time, and the Zn2+ concentrations were determined in the remaining solutions.
Results and discussion
Effect of pH
The adsorption of Zn2+ by leonardite was studied at a pH range of 2-6 for a fixed 0.4 g of leonardite, in order to investigate the optimum pH for the removal of Zn2+. The amount of Zn2+ removed for each pH was calculated by comparing the initial concentrations of Zn2+ with that remaining in the solution after 24 h. For the analysis of Zn2+ in blank solutions showed that there was no leaching of them from the adsorbent. Fig. 1 shows the removal of Zn2+ versus initial pH for a fixed adsorbent dose and initial concentrations of 5 mg/L and 20 mg/L. As can be seen, the removal of Zn2+ from aqueous solutions by sorption on leonardite was pH dependent. By increasing the pH from 2 to 6 the adsorption increased 43% (for 5 mg/L), and to 40% (for 20 mg/L). It has been reported that at pH 2, the adsorption of Zn2+ on leonardite was practically negligible [8, 9]. The pH effect of adsorption on leonardite can be explained by the surface characteristics of the adsorbent. H+ ions affect metal complexation because they have a great affinity for many complexing and ion-exchange sites. At low pH functional oxidized groups (hydroxyl, carboxyl, phenol, methoxy, etc.) of humic acids are protonated. This phenomenon would be the competition between H+ and zinc ions for sorption on the leonardite surface sites, leading to poor removal of Zn2+. As the pH increases Zn2+ will replace hydrogen ions from the surface of the leonardite and therefore the extent of the adsorption increases [9-11].
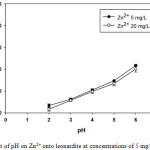 |
Figure 1: Effect of pH on Zn2+ onto leonardite at concentrations of 5 mg/L and 20 mg/L |
Contact time
The percentages of Zn2+ removal against contact time at the optimum pH value 6and with initial concentrations of 5 and 20 mg/L are shown in Fig. 2. Adsorption first follows, an initial rapid process, and the stationary state was observed from 120 min of contact with Zn2+ concentration of 5 mg/L, while at higher initial Zn2+ concentration of 20.00 mg/L, the time necessary to reach equilibrium was after 120 min. For the initial faster rate of metal transition may be explained by the large, uncovered available surface area of the leonardite. The slower adsorption rate in the second phase is probably due to the diffusion of metal ions into the porous structure of the leonardite [1]. If the adsorption materials contain large numbers of oxygen functional groups means that the functional groups act initially as metal coordination sites. However, the experimental data were measured at 180 min to be sure that full equilibrium was achieved.
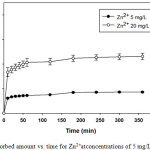 |
Figure 2: Adsorbed amount vs. time for Zn2+atconcentrations of 5 mg/L and 20 mg/L |
Sorption equilibrium: adsorption isotherms
The adsorption isotherm was measured for the initial Zn2+ concentrations ranging from 5.00 to 100.0 mg/L. The tests were carried out for a contact time of zinc ion and 0.4 g L-1 of leonardite dose. The amount of metal ion adsorbed at equilibrium per unit mass of leonardite (qe, mg g-1) was calculated using the following equation:
![]()
where Ci (mg L-1) is the initial metal concentration, Ce (mg L-1) is the final (equilibrium) metal concentration, V (L) is the solution volume and m (g) is the mass of adsorbent. Fig. 3 shows the adsorption isotherm of Zn2+.
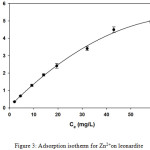 |
Figure 3: Adsorption isotherm for Zn2+on leonardite |
The Langmuir and Freundlich isotherms, which have been extensively used to describe of the adsorption process in different metal-sorbent systems, were used to analyzed the relationship between the adsorption capacity of leonardite and Zn2+ concentrations at equilibriumin the present study. The equations of the Langmuir(Eq. (2)) and Freundlich (Eq. (3)) isotherms are represented as:
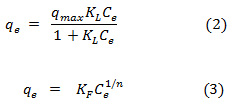
where qmax and KF indicate the adsorption capacity of leonardite, n and KL are related to adsorption intensity. The constant values of the both isotherms can be calculated from the linearized forms of Eqs. (4) and (5) represented by:
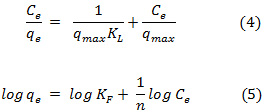
Table 1 and 2 show the calculated Langmuir and Freundlich constants and the corresponding coefficients of correlation values of Zn2+. The experimental data were fitted to the Langmuir isotherm from an equilibrium concentration of 2.08 mg/L (Fig. 4) and the Freundlich isotherm up to an equilibrium concentration 60 mg/L (Fig. 5). According to the Langmuir equation, the maximum adsorption capacities for Zn2+ was 9.19 mg g-1 (0.141 mmol g-1). From Table 2, the Freundlich constant of Zn2+, n, is greater than unity (n = 1.4) indicating favorably adsorption of Zn2+ on leonardite. Furthermore, the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter KR, define as:
![]()
where KR is a dimensionless separation factor. The value of KR indicates the shape of the isotherm to be unfavorable (KR> 1), linear (KR = 1), irreversible (KR= 0) or favorable (0< KR<1) [11-13]. The value of KR for Zn2+is given in Fig. 6. The KR values of Zn2+ is less than 1 indicated that the sorption on leonardite is favorable.
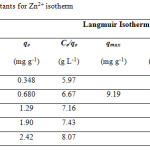 |
Table 1: Langmuir constants for Zn2+ isotherm |
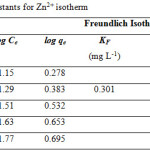 |
Table 2: Freundlich constants for Zn2+ isotherm |
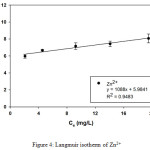 |
Figure 4: Langmuir isotherm of Zn2+ |
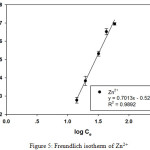 |
Figure 5: Freundlich isotherm of Zn2+ |
Due to the heterogeneous nature of humic substances, the complexation of Zn2+to humic material can be regarding as occurring at the reactive sites with binding affinities that range from weak forces (i.e. ionic) of attraction to formation of highly stable coordinate linkages. In Fig. 7 the structures of types III, IV represent the predominant forms of complexed Zn2+ when humic material are present in abundance. Binding at the weaker sites (I and II) become increasingly important as the stronger sites become saturated. However, explanation of the sorption mechanism is unlikely to be so simple, because of the complex heterogeneous nature of leonardite, together with the abundance of functional groups present in leonardite.
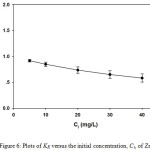 |
Figure 6: Plots of KR versus the initial concentration, Ci, of Zn2+ |
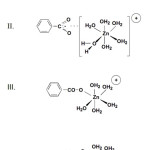 |
Figure 7: Example of Zn2+ binding to humic material |
Conclusion
In this study, leonardite from Mae Moh mine could be able to adsorbed Zn2+aqueous solution. The adsorption in these systems is pH dependent, and the best results were obtained in the pH range 5-6. It would indicate that the ion exchange and metal complexation reaction took place in the metal removal with leonardite. The experimental data in the linear form of Langmuir and Freundlich equations gave satisfactory correlation coefficients and concentration ranges. The average maximum adsorption capacity was 0.141 mmol g-1. The advantage of leonardite sorbent in this study is that it can be used in crude form without pre-treatment step, low cost and is available in large quantities, which makes leonardite a promising candidate for wastewater treatment and further development improved.
Acknowledgements
The authors would like to thanks the Mae Moh Mine in Lampang province, Thailand for leonardite, the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on High Education, Ministry of Education as well as the Graduate School, Chiang Mai University for financial support. This work was also supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
References
- Solé, M., Casas, J.M. and Lao, C., Water, Air and Soil Pollut., 144, 57-65 (2003).
- Al-Degs, Y., Khraisheh, M. A. M. and Tutunji, M. F., Water. Res., 35, 3724-3728 (2001) .
- Janoš, P., Sypecká, J., Mlčkovská, P., Kuráň, P. and Pilařová, V., Sep. Purif. Technol., 53, 322-329 (2007).
- Janoš, P., Šedivý, P., Rýznarová, M. and Grötschelová, S., Chemosphere, 59,881-886 (2005).
- Anđelković, T., Perović, J., Blagojević, S., Purenović, M., Nikolić, R., Bojić, A.and Anđelković, D., Bull. Chem. Technol. Macedonia, 25, 131-137 (2006).
- Hoffman, G.L., Nikols, D.J., Stuhec, S. and Wilson, R.A., Evaluation of leonardite (humalite) resources of Alberta, Energy, Mines and Resources Canada (1993).
- Janoš, P., Lesný, J., Závodská, L., Kříženecká, S. and Herzogová, L.,Nova Biotechnologica, 7, 85-91 (2007).
- Arpa, C., Başyilmaz, E., Bektaş, S., Genç, Ö. and Yürüm,Y., Fuel Process. Technol., 68, 111-120 (2000).
- Pehlivan, E. and Arslan, G., Fuel Process. Technol., 88, 99-106 (2007).
- Pehlivan, E., Energ. Source Part A, 28, 447-457 (2006).
- Ho, Y.S., Huang, C.T. and Huang, H.W., Process. Biochem., 37, 1421-1430(2002).
- Hameed, B.H., J. Hazard. Mater., 162, 344-350 (2009).
- Lewinsky, A.A. Hazardous materials and wastewater: treatment, removal and analysis, Nova Science Publishers, New York; 291-295 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









