A Novel Synthesis and Antimicrobial Studies of 3-(4-Substitutedthiocarbamidophenyl)-N, N-dimethyl-3-Pyridin-2-yl-propan-1-amine
Dipak Tayade1 , Rupesh Shekar2 and Rahim Shekar1
1Department of Chemistry, Mahatma Fule Arts & Commerce & Sitaramji Chaudhri Science Mahavidyalya Warud, Amravati (M.S.) Pin code 444 906 (India).
2Dept. of chemistry, Government, V.I.S.H, Amravati, 444 604 (India).
Heteroacyclic and Heterocyclic containing drugs showed remarkable and noticeable drug absorption, transmission and drug effects; hence they created their own identity and importance in pharmaceutical, medicinal, agricultural and drug sciences. Thioamido and benzamido heterocyclic compounds showed various significances and applications in industrial, pharmaceutical, medicinal and drug chemistry. Considering all these facts into consideration it was thought interesting to synthesize 3-(4-substitutedthiocarbamidophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine by interacting 3-(4-chlorophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine with various thiourea in isopropanol medium .The justification and identification of the structure of these newly synthesized compounds had been established on the basis of chemical characterization, elemental analysis, and through spectral data .
KEYWORDS:Substitutedthiocarbamides, 3-(4-substitutedthiocarbamidophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine and isopropanol
Download this article as:| Copy the following to cite this article: Tayade D, Shekar R, Shekar R. A Novel Synthesis and Antimicrobial Studies of 3-(4-Substitutedthiocarbamidophenyl)-N, N-dimethyl-3-Pyridin-2-yl-propan-1-amine. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Tayade D, Shekar R, Shekar R. A Novel Synthesis and Antimicrobial Studies of 3-(4-Substitutedthiocarbamidophenyl)-N, N-dimethyl-3-Pyridin-2-yl-propan-1-amine. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23970 |
Introduction
Recently in this laboratory the synthetic applications of cynoguanidine and 1, 3-diformamido-thiocarbamide had been briefly explored1. As evident from structure of 3-(4-substituted thiocarbamidophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine, it possesses chloro, amino, methyl and ethyl reactive sites for various reactions. As a wider programmee of this laboratory in the synthesis of nitrogen, sulphur and nitrogen and sulphur containing heteroacycles and heterocycles the interactions of cynoguanidine with various thiourea and isothiocynates have been investigated in sufficient details 2-8. Some of these compounds showed remarkable pharmaceutical and biological activities9-12.
An exhaustive literature survey on thiocarbamids3-6 and 3-(4-substituted thiocarbamido phenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine13-15 showed that these nucleus containing drugs play an important role in pharmaceutical, medicinal and drug chemistry having remarkable pharmaceutical, medicinal and biochemical applications, by considering all these facts into considerations interactions of 3-(4-chlorophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine with various thiourea in isopropanol medium were investigated to isolate yet new series of 3-(4-substitutedthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine.(Scheme 1)
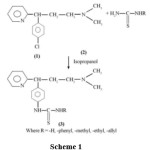 |
Scheme 1 Click here to View Scheme |
For investigating the medicinal and pharmaceutical applications, the antimicrobial activities of synthesized compounds were studied.
Experimental
The melting point of the all synthesized compounds was recorded using hot Paraffin bath. The carbon and hydrogen analysis were carried out on Carlo-Ebra 1106 analyzer. Nitrogen estimation was carried out on Colman-N-analyzer-29. IR spectra were recorded on Perkin Elmer Spectrometer in range 4000-400 cm-1 in KBr pellets. PMR spectra were recorded on Bruckner Ac 300 F Spectrometer with TMS as internal standard using CDCl3 and DMSO–d6 as solvent. The purity of compound was checked on silica Gel-G Pellets by TLC with layer thickness of 0.3 mm. All chemicals used were of AR-grade.
3-(Thiocarbamidophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine (3a):-
A mixture of 3-(4-chlorophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine (1) (0.1M), thiourea (2a) and isopropanol (40ml) was refluxed on boiling water bath for 4 hrs. During boiling suspended 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine went into the solution and the new product was found to be gradually separated out ,which on basification with dilute ammonium hydroxide afforded white crystals. It was filtered in hot conditions and recrystallized with aqueous ethanol to obtained (3a), yield 67.7%, melting point 1580 C. (D)
Properties
It is white, crystalline solid having melting point 1580C. (D). It gave positive test for nitrogen and sulphur. Desulphurised with alkaline plumbite solution. It formed picrate, melting point 1100 C. Elemental analysis: – C [(found 69.81%) calculated 70.76%], H [(found 6.27%) calculated 6.66%], N [(found 14.21%) calculated 14.35%], S [(found8.18%) calculated 8.20%]. IR Spectra:-The IR spectra was carried out in KBr pellets and the important absorptions can be correlated as, (cm-1) 3393.6 (N-H stretching), 2362.7 [C-H (Ar)] stretching, 1661.6 (C-N stretching), 1101.6 (=C=NH imino), 517.3 (N=C=S). PMR Spectra:-The spectrum was carried out in CDCl3 and DMSO-d6 .This spectrum distinctly displayed the signals due to Ar-H, protons at δ7.941-8.54 ppm. Ar-NH protons at δ 6.85 ppm, pyridino-NH at δ 3.97 ppm. –CH2 protons at 2.12-2.86 ppm. –CH3 protons at 1.27 ppm.
Similarly, 3-(4-phenylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine(3b),3-(4-methylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine(3c),3-(4-ethylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine(3d) and 3-(4-allylthiocarbamidophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine(3e) were synthesized by interacting phenylthiourea(2b), methylthiourea(2c), ethylthiourea(2d) and allylthiourea (2e) with 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine(1) in isopropanol medium by above mentioned method and are mentioned in Table No.1.
Table 1
| Sr.No. | Expt.No. | “3-(4-Substitutedthiocarbamidophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine.” | Yield% | m.p. 0C |
| 3b | 2 | …………………… –Phenyl-…………………. | 67 | 171 |
| 3c | 3 | ………………..-Methyl- ………………… | 75 | 167 |
| 3d | 4 | ……………….-Ethyl-………………….. | 78 | 180 |
| 3e | 5 | ………………..-Allyl-…………………… | 65 | 178 |
Antimicrobial And Antifungal Activities:-
The antimicrobial and antifungal activities16 of all these compounds were screened by using cup-plate agar diffusion method in DMF, using standard Co-Trimazin 25 µg/ml against gram positive and gram negative bacteria such as E. coli, S. typhi, S. abony, P. aeruginosa, and B. subtilis. All compounds were also screened for their antifungal activities by using standard Greseofulvin (10µg/ml) against A. niger and C. albicans.
Cup-plate method
A medium used throughout the experiment was HI-Media (India make) having composition of [Pepton-5gm/lit, NaCl -5gm/lit,Yeast extract -1.5gm/lit, Agar powder -20gm/lit, pH – 7.4 ± 0.1]
The medium used for antibacterial and antifungal activities were prepared [N-agar for bacterial and Sabourands dextrose agar for fungi] by dissolving 26 gms of ingredients in one liter of distilled water and sterilized in autoclave at 121 0C at 15 Ibs/inch pressure in an autoclave for 154 minutes. Then microbes were inoculated with requisite quantity to the medium at temperature 40-500 C and immediately poured the inoculate medium into sterilized petridishes to give a depth of 3-4 mm of uniform thickness. After solidification the well or holes were prepared by well borer. The dimethylformamide solution of the compound was added in sufficient amount to fill the well. Then it was kept at room temperature for 4 hrs as a pre-incubation and then plates of bacteria were inoculated for 18-24 hrs, at 36-380C and all plates fungi were inoculated 48 hrs at 20-25 0C. After the period of inoculation, zones of inhibition were recorded around the wells. The results are cited in Table No.2
Table 2
| Comp. No | S.typhi (mm) | E.coli(mm) | S. abony (mm) | P.aeruginosa(mm) | B. subtilis(mm) | A. niger (mm) | C. albicans (mm) |
|
3a |
1.3 |
1.1 |
1.2 |
0.9 |
0.5 |
— |
— |
|
3b |
1.7 |
1.3 |
1.8 |
1.2 |
1.3 |
0.4 |
— |
|
3c |
1.5 |
1.4 |
1.1 |
0.8 |
0.7 |
— |
— |
|
3d |
1.2 |
0.8 |
0.6 |
0.9 |
0.6 |
0.2 |
— |
|
3e |
1.3 |
1.1 |
1.3 |
1.2 |
0.7 |
— |
— |
All the seven organisms studied are human pathogens; from the results it is clear that all the synthesized compounds showed remarkable and considerable antimicrobial activities. These thiocarbamides showed highly activity against E. coli, S. typhi, S. abony, P. aeruginosa, B. subtilis, While less active against A. niger and C. albicans. Hence study of these compounds is required in biochemical and medicinal directions. From the above data it is concluded that this compounds showed remarkable antibacterial activity that antifungal activity. S. typhi causes typhoid while E. coli causes diarrhoea and S. abony causes pus formation. It is observed from literature survey of medicinal sciences that in the last two decades the patients of typhoid and diarrhoea throughout the world are common. Lower drugs of typhoid are now totally rejected and higher drugs are now given to the patients. As newly synthesized thiocarbamides showed remarkable and considerable activities so these compounds can be used as alternative for the treatment of diseases caused by the above mentioned pathogens only if they do not have toxic and other side effects after the details study. The potency of the drug is increased due to substitution of thiocarbamido moiety on the previous drug.
References
- Tayade D. T, Ph.D Thesis Amra,vati University, Amravati 1996.
- Rudnitskaya O. V, Kultyshkina E. K, Linko I. V, Sergienko V. S and Aleksandrov C. G, Russian J of Co- ordination Chem 2010, 36, 137-142.
- Tayade D.T., Bhagwatkar R.A, Panpalia R.C., International Journal of Chemistry Canada.2010 2 (2), 41-43
- Panpalia R.C. ‘studies in the chemistry of some thiocarbamide’s and Hector’s bases’ Ph.D Thesis,SGB Amravati University Amravati 2006
- Bhagwatkar R.A , Tayade D. T. , Orbital Elec. J. Chem., Campo Grande Brazil, 2011, 3(1), 53-56.
- Tayade D.T.Pund .D.A., Bhagwatkar R.A, Rathod D.B. Bhagwatkar N.A., International Journal of Chemistry Canada 2011, 3 (1), 36-41.
- Tayade D.T., Raghuwanshi M. R., Bhagwatkar R.A., International Journal of Chemistry Canada 2011 , 3 (2), 74-78.
- Shrivastava P.K., “Bases related with thiourea”, Ph.D. Thesis , B.H.U. 1964.
- Dover L.G, Alahari A., Gratraud P, Gomes J. M, Bhowruth. V, Reynolds R. C, Besra G. S and Kremer Ln, Antimicrobial Age ts and Chemotheraphy 2007, 519(3), 1055-1063.
- Paranjpe M.G, J Indian Chem Soc 1999, 42, 45.
- Joshua.C.P. “Chemistry of Hector’s base”,Ph.D., Thesis, B.H.U 1962
- Deohate P.P., Berad B.N., Indian J.Chem.2005, 44B,638-642.
- www.drugs.com
- Waghmare.J.S Ph.D Thesis S.G.B. Amravati University, Amravati 2007
- Panpalia R.C. Ph.D Thesis S.G.B. Amravati University, Amravati 2005
- Raghuwanshi M.R. Ph.D Thesis S.G.B. Amravati University, Amravati 2009

This work is licensed under a Creative Commons Attribution 4.0 International License.









