A New Tetrahydropyrano[3,4-c]pyran-1(3H)-one Iridoid from Viburnam cylindricum
Dwarika Prasad¹* and S. P. Sati²
1Department of Chemistry, Lovely Professional University, Punjab (India).
2P. G. College, Gopeshwar Chamoli, Uttrakhand (India).
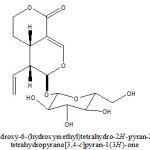
From ethanolic extract of Viburnam cylindricum plant a (4aS, 5R, 6S)-6-(3,4,5-trihydroxy-6(hydroxymethyl) tetrahydro-2H-pyran-2-yloxy)-5-vinyl-4,4a,5,6-tetrahydropyrano[3,4-c]pyran-1(3H)-one Iridoid have been isolated and characterized with help of 1H, 13C NMR, 1H-1H COSY, DEPT and HMQC studies. This is new studies in chemical analysis of Viburnam cylindricum.
KEYWORDS:Viburnam cylindricum; Capriofoliaceae; (4aS,5R,6S)-6-(3,4,5-trihydroxy-6(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-5-vinyl-4,4a,5,6-tetrahydropyrano [3,4-c]pyran-1(3H)-one
Download this article as:| Copy the following to cite this article: |
| Copy the following to cite this URL: |
Introduction
Viburnum cylindricum belong to the family Capriofoliaceae evergreen shrubs with grey bark, leaves oblong lanoceolate or ovate glaucous green above occurs in moist shaded oak forest 1200-2500 mt. [1] From leaves of V. Cylindricum Neochlorogenic acid methyl ester, cryptochlonogenic acid ester and chlorogenic acid methyl ester are isolated[2]. From leaves of V. Pronifolium 2- acetyldihydropenstemide, 2′- trans-p-caumrayl dihydropenstemide, 2- acetylpatrinoside and patrinosid are isolated[3]. From leaves of V. dilatatum p- hydroxyphenyl-6-0-trans-caffeoyl-b-D-glucoside, p-hydroxyphenyl-6-O-transcaffeoyl- b-D-apiosyble [1-6]- b-D-glucoside are isolated[4]. From leaves of V. orientale Acyclic monoterpendiglycosides was isolated[5]. The structure of compounds have been elucidated through. 1H, 13C NMR and 2 D-NMR spectra and biological activities of plant extract.
Result and discussion
It was obtained as yellow amorphous powder from methanol. The elemental analysis of compound found values, C=53.58%, H=6.11%, required values for C16H22O9; C=53.63%, H=6.14%, Molecular weight 358. UV- spectrum of compound showed characteristic absorption bands at 244 nm for an showed a typical iridoid enol ether system conjugated with a C-4 carbonyl group (6). Compound showed a typical iridoid colour reaction with hydrochloric acid.
1H- NMR (400MHz, CD3OD): 5.43 (1H, d, J=1.2Hz, H-1), 7.56(1H,d,J=2.4 Hz, H-3), 3.11 (1H, m, H-5), 1.71(2H, m, H-6), 4.41 (2H, m, H-7), 5.56 (1H, m, H-8), 2.72 (1H, m, H-9), 5.33 (1H,dd, J=2.0, 16.8 Hz, H-10a), 5.25 (1H, dd, J=2.0, 9.0 Hz, H-10b), 4.63 (1H,d, J=8.0 Hz, H-1’), 3.22 (1H, dd, J=80, 8.8 Hz, H-2’), 3.71 (1H, t, J=8.8Hz, H-3’), 3.31 (1H, m, H-4’), 3.44 (1H, m, H-5’), 3.65 (1H, dd,J=12.0, 6.0 Hz, H-6’a), 4.02 (1H, dd, J=12.0, 6.4 Hz, H-6’b). The detail analysis of 1H-NMR spectrum, coupled with of 1H-1H COSY indicated presence of two methylene protons at 1.71 ( multiplet for two protons, H-6) which showed coupling with a methylene protons signals (multiplite for two protons) resonated downfield ( 4.41, H-7) in comparison with H-6 indicated that the later is attached with an oxygen function. The methylene proton signal at 1.71 also showed coupling with a methine proton signal at 3.11 (m, 1H, H-5) which in turn showed coupling with a methine proton signal at 5.43 (d, J=1.2 Hz, H-1) and 2.72 (1H, m, H-9). Detailed analysis of 1H-1H COSY showed that the methine proton signal attached to a mono-substituted double bond resonance at 5.56 (1H, m, H-8) showed coupling with a methine proton appeared at 2.72 (H-9), and with two methylene protons appeared at 5.33 (1H, dd, J=2.0, 16.8 Hz, H-10a), and 5.25 (1H, dd, J=2.0, 9.0 Hz, H-10b). These data indicated presence of a vinyl group in the molecule. An integrated methine proton signal which appeared as a double (J=2.4) at 7.56 showed long range coupling in 1H-1H COSY spectrum with the metine proton signal appeared as a multiplet at 3.11 A doublet (J=1.2 Hz) appeared at 5.43 was corroborated with the H-1 proton signal of most of the iridoids having O-glycosylation at C-1 carbon (7-9). Beside these protons signals the 1H- NMR showed a doublet at 4.63 (1H, d, J=8.0 Hz, H-1’), which was corroborate with the presence of a beta-D-glucose moiety in the molecule. The above discussed 1H- NMR data are stongly reminiscent with those reported for sweroside (10-12).
13C- NMR (100 MHz, CD3OD); 98.1 (C-1), 154.3 (C-3), 105.3 (C-4), 27.3(C-5), 25.1(C-6), 70.1(C-7), 132.1(C-8), 42.6(C-9), 121.2(C-10), 169.7(C-11), 99.4(C-1’), 73.7 (C-2’), 76.2 (C-3’), 70.2(C-4’), 72.1(C-5’), 61.0 (C-6’). The signals out of which 2 are quaternary, 10 methine and four methylene carbons. The assignment of methylene, methine and quaternary carbon signals readily made by HMQC experiment.
Acid hydrolysis of compound
Hydrolysis of compound was carried out similar to that of compound and the sugar was identified as D-glucose.
The glycoside nature of compound was supported by a doublet at 4.63 (J=8.0 Hz) assiggable to the anomeric proton of beta D-glycose. The 13C-NMR chemical shift of anomeric carbon atom (C-1’) at 99.4 and the chemical shift of other carbon atom of sugar moiety (see experimental) are in agreement with the 1H-NMR spectrum and thus confirmed presence of glucose in molecule. The usual location of sugar moiety at position O-1 of the aglycone was showed by the downfield shifted signal of 1H (5.43).
The 13C-NMR data of compound are in agreement with the 1H-NMR data. The presence of a vinyl function as deduced by 1H-NMR was confirmed by 13C chemical shift of unsaturated carbon atom at 132.1 (C-8) and 121.2 (C-10). The 13C-NMR spectrum also showed presence of two methine carbon bearing an oxygen at 70.1 (C-7), a secondary carbonyl carbon [98.1 (C-1)], a tri-substituted double bond [154.3 (C-3), 105.3(C-4)] and a carbonyl function at 169.7. The location of vinyl group was determined at position C-9 by the 1H-NMR spectrum in which a methine proton appeared as a multiplet at 2.72(H-9) showed coupling with three methine protons appeared at 5.43 (1H, d, J=1.2 Hz, H-1), 3.11 (1H, m, H-5) and 5.56 (1H,m,H-8). The UV absorption coupled with the 1H and 13C-NMR data established that a carbonyl group is located at C-4 carbon. On the basis of above discussed spectrum data compound was characterized as sweroside which was confirmed by comparison of spectral data with the reported data (10-12).
Experimental
General experimental procedure
1H-NMR at (400 MHz),13C-NMR at (75 MHz) TMS as internal standard, using DMSO as solvent column chromatography was carried out on silica-gel 60-120 mesh (Merck). TLC was performed on percolated silica-gel. The eluting solvent was CHCl3-MeOH spots were visualized by 7% H2SO4 followed by heating.
Plant material
The whole plant of Viburnam cylindricum were collected from Bacchear District. Chamoli Uttrakhand in the month of October and identified by Department Botany, P.G. College Gopeshwar where vaucher specimen was deposited.
Extraction and isolation
The air dried whole plant (3 kg) was exhaustively extracted with 90% aqueous EtOH for 72 hours. The ethanol extract was concentrated to dryness. The dry ethanolic extract was chromatographic over silica-gel using Methanol Chloroform (70:30) as elution solvent which afforded the compound.
 |
Scheme 1 |
References
- Gaur; R.D. Flora of District Garhwal” Trans Media, Srinagar Garhwal 1999.
- Zurich-AG, Helvetica Chemica. Acta 2005, 88 : 2, 339-342.
- Tomassiru-L, Cometa-MF, Foddai-S, Nicoletti-M, Planta Medica 1999, 65 : 2, 195.
- Machida-K, Nakona-Y, Kikuchi-M, Phytochmistry, 1991, 30 : 6, 2013-2014.
- Calis-I, Yuruker-A, Ruegger-H, Wright-AD, Stricher-U, Helvetica chemical Acta 1993, 76 : 1, 416-424.
- Stuppner, H., Muller, E.P., Maller, E.P., Mathuram, V. and Kundu, A. B., Phytochemistry. 32(2), 375 (1993).
- Takeda, Y., Tsuchida, S. and Fujita, T., Phytochemistry, 26(8), 2303 (1987).
- Stricher, O., Helv. Chem.Acta., 53, 2010 (1970).
- Chaudhari, R.K., Afifi-Yazar, F.U. and Stricher, O., Tetrahederon,36,2317 (1980).
- Battersby, A.R. Hali, E.S and Southgate, E., J. Chem.Sco., 5, 721 (1969).
- Inouye, H., Ueda,S and Nakamura, Y. Tetrahedron Letter, 43, 5229 (1966).
- EI-naggar, L.J and beal, J.L., J Nat.peod., 43.649 (1980).

This work is licensed under a Creative Commons Attribution 4.0 International License.









