A New HPLC Method for Determination of Losartan in Human Plasma and its Application in Bioequivalence Studies
F. Shokraneh1, A. Dabirsiaghi1* and N. Adib2
1Department of Pharmaceutics, Pharmaceutical Sciences Branch, Islamic Azad University (IAU), Tehran, Iran.
²Food and Drug Lab Research Center, Ministry of Health, Tehran (Iran).
Correwsponding Author E-mail: alireza_dabirsiaghi@yahoo.com
A reliable, simple and sensitive reversed-phase high-performance liquid chromatographic method was developed for determination of losartan in plasma .Separation is achieved by HPLC after direct injection on a CN (250*4.6 mm) analytical column with a mobile phase composed of sodium hydrogen phosphate buffer - acetonitrile - tetrahydrofurane - methanol and phosphoric acid (0.1:5 :4 :21 :69.9 ) V/V% adjusted to pH= 9.9. Detection is by ultraviolent absorbance at 254nm. The flow rate was set at 0.6 ml/min. The lower limit of quantitation was 5 ng/ml. The intra and inter-day precisions (CV %) of the quality control samples were 0.57-5.31% and 0.21 -4.52% respectively. The recovery of method was 92.25± 2.19. The method was applied to a bioequivalence study in human.
KEYWORDS:Losartan; Human plasma; HPLC; Bioequivalence
Download this article as:| Copy the following to cite this article: Shokraneh F, Dabirsiaghi A, Adib N. A New HPLC Method for Determination of Losartan in Human Plasma and its Application in Bioequivalence Studies. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Shokraneh F, Dabirsiaghi A, Adib N. A New HPLC Method for Determination of Losartan in Human Plasma and its Application in Bioequivalence Studies. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23799 |
Introduction
The renin-angiotensin (RAS) is a major regulator of blood pressure (BP),1Losartan (COZAAR) is the prototype of a new class of orally active, non –peptide angiotensin II receptor antagonists (ARBs) able to inhibit the renin-angiotensin system specifically and selectively without the agonistic effects of the peptide receptor antagonists.2Approximately 14% of an oral dose of losartan is converted to the 5-carboxylic acid metabolite EXP 3174, which is more potent than losartan as an AT1-receptor antagonist. The metabolism of losartan to EXP 3174 and to inactive metabolites is mediated by CYP2C9 and CYP3A4. Peak plasma levels of losartan and EXP 3174 occur 1–3 hours after oral administration, respectively, and the plasma half-lives are 2.5 and 9 hours, respectively. The plasma clearances of losartan and EXP 3174 (600 and 50 mL/min, respectively) are due to renal clearance (75 and 25 mL/min, respectively) and hepatic clearance (metabolism and biliary excretion). The plasma clearance of losartan and EXP 3174 is affected by hepatic but not renal insufficiency. Losartan should be administered orally once or twice daily for a total daily dose of 25–100 mg.3 Last studies showed that several HPLC ,LC/MS/MS methods were used for determination of losartan and its metabolite in human plasma.4-12The aim of this study was to develop a simple, rapid sensitive and reliable HPLC method with Ultraviolent detection for quantization of losartan in human plasma samples and to compare the bioavailability of two losartan tablets (50 mg) formulations (losartan from Iranian company, as a test formulation and COZAR, MSD ,The Netherlands as a reference formulation) . The method was validated according to procedures and acceptance criteria based on FDA guideline and recommendations of ICH, to provide enough selectivity, sensitivity and reliability in pharmacokinetic and bioequivalence studies.
Materials and Methods
Hydrochloric acid, methanol (HPLC grade) ,ammonia ,phosphoric acid, sodium hydrogen phosphate ,acetonitrile ,tetrahydrofurane , were purchased from Merc. Losartan and Thioridazine were USP reference standard.
Sample and standard solutions preparation:
Blood samples were collected from individuals and centrifuged in order to separate the plasma. (Separated plasma was stored at -20 °C.). To a 0.5 ml aliquot of plasma, 1ml of internal standard (thioridazine) was added. 500 µl of 0.2 M HCL was used to acidify the solution and then vortexed and transferred in to the cartridge (Bond ElutTM) CN which was prepared by using pure methanol and water in a proportion of 2:1. Initially 500µl distilled water and 10% methanol followed with 50µl of pure methanol was put through the cartridge. The internal standard and drug was then collected. The pH of the solution was made alkaline (pH =8) using ammonia/methanol in a proportion of 1:99 respectively and then dried under Nitrogen. The mobile phase was then poured in to the product in order to reduce it (The product was then reduced using the mobile phase (100µl)). 80µl of this solution was injected in to the HPLC with UV detector with the flow rate of 0.6ml/min and the column of CN (250*4.6mm). Stock solution of losartan (25 to 5000ng/ml) was prepared and diluted by blank plasma to obtain the final concentration (2.5 to 500ng/ml) of Losartan curve. Data from HPLC in defined circumstances was analyzed and evaluated.
Instrument and chromatographic conditions:
Analyses were performed on younglin model ACME-900 pump equipped with Ultraviolet detector at wavelength of 254nm. Chromatography was performed at room temperature on a CN column (250*4.6mm). The mobile phase consisted of sodium hydrogen phosphate buffer – acetonitrile – tetrahydrofurane – methanol and phosphoric acid (0.1:5:4:21:69.9) V/V% adjusted to pH 9.9 using phosphoric acid. The flow rate was set at 0.6ml/min respectively.
Method validation
The method validation demonstrated the specificity, lower limit of quantification (LOQ), recovery, linearity, precision and accuracy of measurements.13
Specificity was investigated by analyzing six drug-free plasma samples for interference of endogenous compounds. For calibration curve five different concentrations of losartan (2.5 to 500ng/ml) in plasma were prepared by adding required volume of working solutions to blank plasma. Plasma calibration curve was prepared by taking area ratio of analyte to internal standard as Y-axis and concentration of analyte (ng/ml) as X-axis. Linearity of the standard curve was evaluated using least squares linear regression analysis. The limit of quantification (LOQ) was taken in this work at the lowest concentration standard affording accuracy and precision ≤20%, using five plasma samples. The intra and inter-day precisions (CV %)of the assay procedure were determined by trice analysis of quality control plasma samples (50 ,200 and 500 ng/ml) at the someday and three different days. Recovery was determined by comparing the response of three pre-treated quality control plasma samples in three levels (25, 100 and 250 ng/ml) with the absolute peak area of un-extracted samples containing the same concentration of the drug as 100%.
Application
The validated method was used in bioequivalence study of losartan. It was an open, one-centre, cross-over ,randomized, three way and double-blind study to asses relative bioavailability of losartan in twenty-four healthy volunteers following single dose administration of losartan as 50 mg tablet (All subject gave informed consent to the work). The reference (COZAR, MSD, The Netherlands) and test (manufactured by Iranian company) product were used in this study. The blood collecting times were 0 ,0.25 ,0.5 ,0.75 ,1 ,1.5 ,2 ,3 ,4 ,5 ,6 ,8 ,12 ,24 ,36 ,48 hour after oral administration of 50mg losartan reference and test to fasting volunteers. The plasma samples were analyzed by the described method. The pharmacokinetic parameters like area under the plasma-concentration curve from zero to the last measurable losartan sample time and to infinity (AUC o-t and AUC o-inf),maximum concentration (C max) ,time to maximum concentration (T max) were determined for the period of 0-48 hour.
Statistical analysis
The analysis of variance was performed on data for differences between and within the subspecies using the ANOVA (SPSS ver.10). mean separation were determined by least significant difference (LSD) at P≤0.05% .
Results And Discussion
Some HPLC and LC-MS-MS methods have been developed for determination of losartan. The proposed method is suitable for losartan quantification in plasma samples. The based on a simple liquid – liquid extraction (LLE) followed by high – performance liquid chromatography with UV detector, using thioridazine as an internal standard, respectively. Blood samples were collected at specified time intervals, and the plasma separated and analyzed for losartan. Blank plasma and spiked plasma with losartan and internal standard are shown in figure 1. Retention time for the losartan and internal standard were 3.5 min and 8 min. The chromatograms also confirmed the complete separation. The calibration curve could be described by the equation:
Conc = 77area% + (-6.1), r2 =0.9921
The assay exhibited a linear dynamic range of 2.5 – 500 ng/ml, a run time of 20 min for each sample made it possible to analyze more than 70 samples per day. The limit of quantitation (LOQ ) was 5 ng/ml. Intra and inter-day precisions (CV%) of the quality control samples were 0.57 – 5.31% and 0.21 – 4.52% ,the accuracy of this bioanalytical method was 102.85±2.33 respectively (Table 1). The overall intra and inter assay variations were within acceptance limits according to FDA guidelines.
The plasma samples from 24 health human volunteers were assayed with the validated method described above. The mean concentration – time curve is shown in figure 2.
Maximum plasma concentration (C max) ranged from 104.53 to 176.44 ng/ml for test product and 121.03 to 192.60 ng/ml for reference product at 1.5 to 4 hour (T max). Also the mean value of area under the concentration time curve (AUC o-t) obtained was 1485.60 ng h/ml, AUC o-inf was found to be 2397.45 ng h/ml . No statistical differences were observed for C max, T max, AUC o-infand other pharmacokinetic parametersand the test formulation is bioequivalent to the reference formulation for losartan.
The paper describes a rapid and reproducible HPLC method which enables the determination of losartan in plasma.
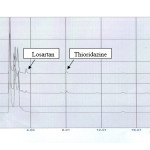 |
Figure1. Chromatograms of (1) Blank human plasma; (2) Plasma spiked with thioridazine (rt=3.5min) and losartan (rt=8.0 min); (3) Human plasma after administration of losartan tablet |
(Table 1: Precision and Recovery of Losartan Assay in Human Plasma (n=3
|
Spiked (ng/ml) |
Intra-day |
Inter-day |
Spiked (ng/ml) |
Recovery% |
||||
|
Found |
SD |
CV% |
Found |
SD |
CV% |
|
||
|
50 |
50.30 |
6.02 |
11.51 |
55.66 |
2.51 |
4.52 |
25 |
89.99 |
|
200 |
207.30 |
11.01 |
5.31 |
205.66 |
2.51 |
1.22 |
100 |
94.37 |
|
500 |
501.10 |
2.85 |
0.57 |
501.46 |
1.05 |
0.21 |
250 |
92.38 |
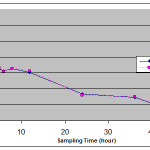 |
Figure 2: Mean plasma concentration – time curve for test and reference preparation following single oral administration of losartan 50 mg tablet in 24 healthy volunteers |
The main advantage of this method is the use of precipitation for purification, which is easily and fast in comparison with other purification and extraction methods. This HPLC method is reliable, reproducible and sensitive with respect to validation parameters. It can be used as an assay method in the study of losartan pharmacokinetics as well as bioavailability/ bioequivalence studies.
References
- Weir MR. Clinical Therapeutics., 29: 1803-24 (2007).
- Timmermans PB, Wong PC, Chiu Atand Smith RD. J Hypertens Suppl., 13: 1-13 (1995).
- Joel G Hardman, Lee E Limbird, Alfred Goodman Gilman(2001).Goodman & Gilmans The pharmacological Basis of therapeutics,tenth ed. USA,829-833.
- Jia JY, Zhang MQ. Liu YM, Liu Y, Liu GY, Li SJ, Lu C, Weng LP, Qi YL and Yu C. Clinical Therapeutics., 37:1387-95 (2010).
- Koytchev R, Ozalp Y, Erenmemisoglu A, Van der Meer MJ and Alpan RS. Arzeimittelforschung., 54: 611-7 (2004).
- Tamimi JJ, Salem II, Mahmood Alam S, Zaman Q and Dham R. Biophram Drug Dispos., 26: 205-10 (2005).
- Oliveira CH, Medeios Silva R, Santagada V, Caliendo G, Perissutti E, Prado Galuppo M, Marcondes Rezende V, Barrientos-Astigarraga RE, mendes GD and De Nucci G. Int J Clin Pharmacol Ther., 44: 142-8 (2006).
- Bienert A, Brzeziniski R, Szalek E, Dubai V, Grzeskowiak E, Dyderski S, Drobnik L, Wolc A and Olejniczak-Rabinek M. Arzeimittelforschung., 56: 723-8 (2006).
- Kolocouri F, Dotsikas Y, Apostolou C, Kousoulos C and Loukas YL. Anal Bioanal Chem., 387: 593-601 (2007).
- Neves R, Almeida S, Filipe A, Spinola AC, Abolfathi Z, Yritia M and Ortuno J. Arzeimittelforschung., 58: 369-75 (2008).
- Salvadori MC, Moreira RF, Borges BC, Andraus MH, Azevedo CP, Moreno RA and Borges NC. Clin Exp Hypertens., 31: 415:27 (2009).
- Prasaja B, Sasongko L, Harahap Y, Hardiyanti, Lusthom W and Grigg M. J Pharm Biomed Anal., 49: 862-7 (2009).
- Adib N., Shekarchi M., Dabirsiaghi A., Hajimehdipoor H., Rastegar H. and Akbari-Adergani B., Bioscience, Biotechnology Research Asia., 7/2 : 603-606 (2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









