A Green, Reusable and Highly Efficient Heterogeneous Catalyst for the Synthesis of Arylpyrazoles using Nano-Fe2O3
Farhad Hatamjafari*
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Corresponding Author E-mail: f_hatamjafari@tonekaboniau.ac.ir
Series of some new arylpyrazole derivatives have been synthesized in good yields via one-pot condensation reaction using nano-Fe2O3 as heterogeneous catalyst.
KEYWORDS:Arylpyrazole; Nano-Fe2O3; solvent-free; Baylis-Hillman
Download this article as:| Copy the following to cite this article: Hatamjafari F. A Green, Reusable and Highly Efficient Heterogeneous Catalyst for the Synthesis of Arylpyrazoles using Nano-Fe2O3. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Hatamjafari F. A Green, Reusable and Highly Efficient Heterogeneous Catalyst for the Synthesis of Arylpyrazoles using Nano-Fe2O3. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23770 |
Introduction
Recently, organic reactions catalyzed by nanoparticles (NPs) have attracted much attention. Consequently, there is a great demand for development of novel catalysts with higher catalytic activities, lower prices, good recyclability and less pollute to the environment in their catalytic systems. Many organic reactions occur more efficiently in the solid-state than in solution and in many cases even more selectively, because molecules in the crystals are arranged tightly and regularly. Nanoparticles have emerged as sustainable alternatives to conventional materials, as robust high–surface-area heterogeneous catalyst supports[1].A magnetic nanoparticle catalyst was readily prepared from inexpensive starting materials which catalyzed the Hantzsch reaction. High catalytic activity and ease of recovery from the reaction mixture using an external magnet, and several reuse times without significant losses in performance are additional eco-friendly attributes of this catalytic system[2].
Arylpyrazole is an important class of organic compounds, which received a considerable attention due to their wide range of biological activities and widely used as pharmaceuticals, agrochemicals, anti-inflammatory, antiviral, antibacterial[3-8]. Although heterocycle compounds are valuable compounds and many applications have been reported[9].
Previously, we have synthesized a number of heterocyclic compounds[10-13]. In our ongoing research prompted by our interest in multiple component reactions and as part of programs in the area of heterocyclic compounds containing nitrogen[14], and due to the resultant pharmacological interest in compounds which belong to the diarylpyrazoles, although this reaction done previously in other conditions[14-16], herein we report in a different condition one pot reaction with high yields and easy separation of product for the construction of some 1, 5-diarylpyrazole derivatives, via condensation of Baylis-Hillman adduct and phenyl hydrazine Using nano- Fe2O3 (Scheme 1).
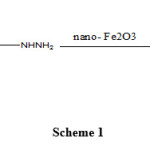 |
Scheme 1 |
Baylis-Hillman adducts were prepared by the reaction of ethyl vinyl ketone, arylaldehydes[17]. For synthesis of 1, 5-diarylpyrazole derivatives, the reaction of Baylis-Hillman adduct (1), phenylhydrazine hydrochloride in 1,2-dicloroethane Using nano-Fe2O3 was used (Scheme 1). Therefore preparation of all the 1, 5-diarylpyrazoles described in this paper, the reaction was complete within 30-40 min on solid support nano-Fe2O3 in excellent yields (78-88%) to afford 4a-e (Table 1).
Table 1: Three-component synthesis of some 1, 5-diarylpyrazoles
|
Entry |
R |
Ar |
Yields (%) |
Time(Min) |
IR ν C=N cm–1
|
1H-NMR (CDCl3) δ, ppm |
|
|
4a |
-Me |
phenyl |
78 |
30 |
1610 |
1.36 (t, J = 7.6 Hz, 3H), 1.94 (s, 3H), 2.75 (q, J = 7.6 Hz, 2H), 7.21-7.26 (m, 5H), 7.34 (dd, J = 7.6, 1.4 Hz, 1H), 7.55 (dt, J = 8.1, 1.4 Hz, 1H), 7.62 (dt, J = 7.5, 1.30 Hz, 1H), 7.98 (dd, J = 8.1, 1.2 Hz, 1H) |
|
|
4b |
-Et |
o-nitrophenyl |
82 |
40 |
1615 |
1.38 (t, J = 7.5 Hz, 3H), 1.95 (s, 3H), 2.7 (q, J = 7.5 Hz, 2H), 7.20-7.26 (m, 5H), 7.33 (dd, J = 7.5, 1.5 Hz, 1H), 7.58 (dt, J = 8.5, 1.5 Hz, 1H), 7.65 (dt, J = 7.5, 1.30 Hz, 1H), 8.0 (dd, J = 8.5, 1.3 Hz, 1H) |
|
|
4c |
-Et |
p-nitrophenyl |
83 |
32 |
1600 |
1.34 (t, J = 7.2 Hz, 3H), 2.12 (s, 3H), 2.79 (q, J = 7.2 Hz, 2H), 7.20 (dd, J = 8.5, 1.3 Hz, 2H), 7.30-7.38 (m, 3H), 7.40 (d, J = 8.5 Hz, 2H), 8.30 (d, J = 8.5 Hz, 2H) |
|
|
4d |
-Et |
m-Chlorophenyl |
85 |
35 |
1605 |
1.41 (t, J = 7.5 Hz, 3H), 2.4 (s, 3H), 2.9 (q, J = 7.5 Hz, 2H), 7.3 (dt, J = 7.5, 1.2 Hz, 1H), 7.25-7.35 (m, 8H) |
|
|
4e |
-Et |
p-Chlorophenyl |
88 |
36 |
1609 |
1.38 (t, J = 7.5 Hz, 3H), 2.13 (s, 3H), 2.81 (q, J = 7.5 Hz, 2H), 7.14 (d, J = 7.0 Hz, 2H), 7.20-7.26 (m, 3H), 7.31 (m, 2H), 7.35 (d, J = 7.0 Hz, 2H) |
|
Results And Discussion
Herein, we report synthesis of 1, 5-diarylpyrazole derivatives, the reaction of Baylis-Hillman adduct (1), phenylhydrazine hydrochloride in 1,2-dicloroethane Using nano-Fe2O3 was used (Scheme 1), which could provide an efficient and simple route for the synthesis of diarylpyrazoles and also the required compound obtained this product in a very high yield.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Girija D., Naik H. S., Sudhamani C. N., and B. Vinay Kumar., Arch. Appl. Sci. Res., 3: 373 (2011).
- Koukabi N., Kolvari E., Khazaei A., Zolfigol M. A., Shirmardi-Shaghasemi B., and Khavasi H. R., Chem. Commun., 47: 9230 (2011).
- Lee K. Y. Gowrisankar G. and Kim J. N., Tetrahedron Lett., 46: 5387 (2005).
- Haddad N., Salvagono A., and Busacca C., Tetrahedron Lett., 45: 5935 (2004).
- Lyga J. W., Patera R. M., Plummer M. J., Halling B. P., and Yuhas D. A., Pestic. Sci., 42: 29 (1994).
- Cacchi S., Fabrizi G., and Grangio A., Synlett., 959 (1997).
- Lee K. Y., Kim J. M., and Kim J. N., Tetrahedron lett., 44: 6737 (2003).
- Huang Y. R., and Katzenellenbogan J. A., Org. Lett., 2: 2833 (2000).
- Shridhar A. H., Keshavayya1 J., Joy Hoskeri H., and Shoukat Ali R. A., International Research Journal of Pure & Applied Chemistry., 1(3): 119 (2011).
- Azizian J., Hatamjafari F., Karimi A. R., and Shaabanzadeh M., Synthesis., 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F., and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., 36: 3563 (2006).
- Azizian J., Hatamjafari F., and Karimi A. R., Journal of Heterocyclic Chemistry., 43: 1349 (2006).
- Hatamjafari F., and Montazeri N., Turkish Journal of Chemistry., 33: 797 (2009).
- Peet N. P., Huber E.W., and Huffman J. C., J. Heterocycl. Chem., 32: 33 (1995).
- Hassaneen H. E., Molecules., 16: 609 (2011).
- Mamaghani M., and Dastmard S., ARKIVOC., (ii): 168 (2009).
- Shi M., Jiang J. K., and Li C.Q., Tetrahedron Lett., 43: 127 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









