Synthesis, Characterization, Structural Studies and Biological Activity of a New Schiff Base- Azo Ligand and its Complexation with Selected Metal Ions
Abbas Ali Salih Al-Hamdani¹ and Shayma A. Shaker²
¹Department of Chemistry, College of Science for Women, University of Baghdad, (Iraq). ²Department of Sciences and Mathematics, Universiti Tenaga Nasional (UNITEN), Jalan Kajang- Puchong, 43009 Kajang, Selangor, (Malaysia).
Article Received on :
Article Accepted on :
Article Published : 07 Sep 2011
The new hexadentate Schiff base ligand L [4,4‘(3Z,3‘Z)-3,3‘-(ethane-1,2-diylbis(azan-1-yl-1- ylidene))bis(1,5-dimethyl-2-phenyl-2,3-dihydro-1H-pyrazole-yl-3-ylidene)bis(diazene-2,1-diyl)bis(3- aminophenol)] has been synthesized from condensation of 4-(2-amino-4-hydroxy-phenylazo)-1,5- dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one (azo) with ethane-1,2- diamine. Monomeric complexes with the general formula [M(L)]Cl2 wher M = Co(II), Ni(II), Cu(II)and Zn(II) are reported. The structures of new ligand, mode of bonding and overall geometry of the complexes were determined through IR, UV-Vis, and NMR spectral studies, magnetic moment measurements, elemental analysis, metal content, and conductance. These studies revealed octahedral geometries for all complexes. Complex formation studies via molar ratio and continuous variation methods in DMF solution were consistent to those found in the solid complexes with a ratio of (M : L) as (1 : 1). Hyper Chem-6 program has been used to predict structural geometries of compounds in gas phase. The heat of formation (ΔHf î) and binding energy (ΔEb ) at 298 K for the free ligand and its metal complexes was calculated by PM3 method. Biological activity of the ligand and its metal complexes against several organisms, Escherichia coli and Staphylococcus aureus are reported. Compounds exhibited the high effect of activity. This may be attributed to the impact of both the chelate effect of Schiff base ligand and the role of the metal in these complexes.
KEYWORDS:Hexadentate ligand; Schiff base-Azo; Structural Studies and Biological Activity
Download this article as:| Copy the following to cite this article: Al-Hamdani A. A. S, Shaker S. A. Synthesis, Characterization, Structural Studies and Biological Activity of a New Schiff Base- Azo Ligand and its Complexation with Selected Metal Ions. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Al-Hamdani A. A. S, Shaker S. A. Synthesis, Characterization, Structural Studies and Biological Activity of a New Schiff Base- Azo Ligand and its Complexation with Selected Metal Ions. Available from: http://www.orientjchem.org/?p=11714 |
Introduction
A great deal of work has been reported on the synthesis and characterization of different types of azo Schiff bases. Due to the excellent donor properties of the azo and azomethine groups, these compounds present one important field in coordination chemistry1,2. In addition to their interesting ligational properties, azo Schiff bases and their complexes have important biological and industrial applications. Azo Schiff base and their complexes with transition metal ions are also of importance due to their complexing, catalytieal biological properties3-5. They found to be important as biochemical, analytical and antimicrobial reagent6,7. Complexes of azo compounds also exhibit bacteriostatic and other biochemical activities. Due to the interesting liganting behavior of such system. The aim of this paper is to synthesized, characterized and study the biological activities of the new multidentate Schiff base azo ligand, 4,4`(3Z,3`Z)-3,3`-(ethane-1,2-diylbis(azan-1-yl-1-ylidene))bis(1,5-dimethyl-2-phenyl-2,3-dihydro-1H-pyrazole-yl-3-ylidene)bis(diazene-2,1-diyl)bis(3-aminophenol) L and its metal complexes.
Experimental
All reagents were commercially available and used without further purification. Solvents were distilled from appropriate drying agents immediately prior to use.
Physical measurements
Elemental analyses (C, H and N) were carried out on a Heraeus instrument (Vario EL). Melting points were obtained on a Buchi SMP-20 capillary melting point apparatus and are uncorrected. IR spectra were recorded as CsI discs using a Shimadzu 8300 FTIR spectrophotometer in the range (4000-250) cm-1. Electronic spectra were measured in the region (200-1100) nm using 10-3M solutions in DMF at 25°C using a Shimadzu 160 spectrophotometer. NMR spectra (1H-, 13C-NMR) were acquired in DMSO–d6 solution using Brucker AMX400 MHz spectrometer with tetramethylsilane (TMS) as an internal standard for 1H NMR analysis. Metals were determined using a Shimadzu (A.A) 680 G atomic absorption spectrophotometer. Chloride was determined using potentiometer titration method on a (686–Titro processor– 665Dosimat–Metrohm Swiss). Conductivity measurements were made with DMF solutions using a Jenway 4071 digital conductivity meter at room temperature. Magnetic moments were obtained using a magnetic susceptibility balance (Jonson Mattey Catalytic System Division).
Synthesis of the compound I (4-(2-Amino-4-hydroxy-phenylazo)-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one)
Amixture solution from HCl (37%, 2 ml), ethanol (10 ml) and distilled water (10 ml), was charged with 4-amino-2,3-dimethyl-1-phenyl-3-pyrozoline-5-one ( 0.203 g, 1mmol). An aqueous solution (8 ml) of NaNO2 (0.069 g, 1mmol) was added in drops while maintaining the temperature between 0—5 οC to the mixture. After that the diazonium chloride was added respectively with constant stirring to cold ethanolic solution of 3-amino phenol (0.11 g, 1 mmol), and then solution of 1M NaOH (25 ml) was added to the dark colored mixture. The mixture was stirred for 1 h at 0 οC and acidified with 1 mL of conc. HCl. The brown product formed was suction filtered and recrystallized from ethanol-water and dried.
Synthesis of Ligand (L)
An ethanolic solution (15 ml) of ethane-1,2-diamine ( 0.3 g, 5 mmol) was added to a mixture containing an ethanolic solution (25 ml) of 4-(2-Amino-4-hydroxy-phenylazo)-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one (3.23 g, 10 mmol) and 5 drops of glacial acetic acid. The reaction mixture was heated on water bath at (40-50 oC for 16 h in presence of K2CO3 after the addition of excess of Ethanol (50 ml). A brown solid was formed, and then recrestallised from methanol. The product was dried over anhydrous CaCl2 in vacuum. Yield is 83%, mp 213 oC as shown in Figure 1.
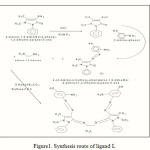 |
Figure1: Synthesis route of ligand L |
General synthesis procedure of the complexes
An ethanolic solution (15 ml) of the metal salt (1 mmol) was added drop wise to an ethanolic solution (20 ml) of the Schiff base ligand L (1 mmol). The resulting mixture was refluxed under N2 for 6 h, resulting in the formation of a solid mass which was crystallized in hot ethanol and dried under vaccum.
Programs used in theoretical calculations
Computational chemistry may be defined as the application of mathematical and theoretical principles to the solution of chemical problems8. Molecular modeling, a subset of computational chemistry, concentration on predicting the behavior of individual molecules within a chemical system. The most accurate molecular models use an initio or (first principles) electronic structure methods, based up on the principles of quantum mechanics, and generally vary computer- intensive. However, due to advances in computer storage capacity and processor performance, molecular modeling has been a rapidly evolving and expanding field, to the point that it is now possible to solve relevant problems in an acceptable amount of time. Electronic structure calculations provide useful estimates of the energetic properties of chemical systems, including molecular structures, spectroscopic features and probable reaction pathways.
Types of Calculation
Single point calculation that determines the molecular energy and properties for a given fixed geometry. Geometry optimization calculations employ energy minimization algorithms to locate stable structures. Vibrational frequency calculations to find the normal vibration modes of an optimized structure. The vibrational spectrum can be displayed and the vibrational motions associated with specific transitions can be animated.
Determination of Bacteriological Activity
Two pathogenic biological, Escherichia coli and Staphylococcus aureus, were used to test the antimicrobial activity of the ligand and its metal complexes. The nutrient agar (NA) medium was prepared and a quantity of 10 ml of the medium was poured in to the sterilized Petri plates and allowed to solidify9. The plates were inoculated with spore suspensions of pathogenic bactericides. By using the sterilized cork borer, wells were dug in the center of the culture plates. The test complexes solution in DMSO was added (0.5 ml) to these wells and the plates were incubated at 25°C for 24 hour .Then the inhibition zone appeared around the wells in each plate was measured and recorded as the cytotoxic effect of the appropriate complexes.
Results and Discussion
The physical analytical data of (L) and its metal complexes are given in (Table 1), which in a satisfactory agreement with the calculated values. The suggested molecular formulae are also supported by subsequent spectral and molar ratio, magnetic moment and conductivity measurements.
Table 1: Physical Characteristics and analytical data for (L) and its metal complexes
|
Compound |
Color |
Yield % |
M.P OC |
Elemental analysis, Calc. (found)% |
||||
|
C |
H |
N |
M |
Cl |
||||
|
(C17H17N5O2) |
Brown |
67 |
175 |
63.15 (63.01) |
5.30 (5.88) |
21.66 (21.05) |
– |
– |
|
(C36H38N12O2) |
Brown |
83 | 213 | 64.46(63.76) | 5.71(6.11) | 25.06(26.42) | – | – |
| [CoL]Cl2 |
Dark green |
67 | >360 | 53.95(55.01) | 4.74(4.12) | 20.98(19.87) | 7.36(6.67) | 8.85(8.01) |
| [NiL] Cl2 | Green | 62 | >360 | 53.97(55.07) | 4.74(5.25) | 20.99(21.56) | 7.33(8.43) | 8.85(8.98) |
| [CuL] Cl2 | DarkBrown | 58 | >360 | 53.65(55.11) | 4.71(5.12) | 20.86(21.11) | 7.89(6.23) | 8.80(7.64) |
| [ZnL] Cl2
|
Brown | 54.7 | >360 | 53.52(54.68) | 4.70(5.48) | 20.81(21.5) | 8.1(7.54) | 8.80(7.28) |
From the wide studied range of molar concentration (10-5-10-3 M) of the mixed solutions, only concentration of (10-4 M) obey Lambert- Beer’s law and showed intense color. A calibration curve was plotted on absorbance against molar concentration in the range (1×10-4-3×10-4M). Best fit straight lines were obtained Figure 2 with correlation factor R> 0.998.
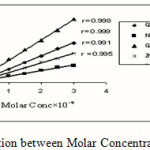 |
Figure 2: Linear Relation between Molar Concentration and Absorbance |
The composition of the complexes formed in solution has been established by mole ratio and job methods. In both cases the results reveals (1:1) metal to ligand ratio.
NMR spectral studies
The newly synthesized ligand gave a satisfactory spectral data and the molecular structure was assigned on the basis of 1HNMR and 13CNMR chemical shift. NMR spectra were determined in solution of (CDCl3) with tetramethyl silane as an internal reference. The identification was using simple splitting patterns that were produced by the coupling of protons and carbons which they have a very different chemical shifts. According to the results obtained from the shift spectra, the molecular structure.
The 1H and 13C NMR spectrum of the complex [ZnL]Cl2 in DMSO-d6 showed peaks of coordinated ligand, which are shifted and observed at; δH (400 MHz, DMSO-d6), 9.7 ppm (2*,2H, δ(OH), 7.54-7.84ppm (2*,16H, δ(CH) aromatic), 4.8 ppm (2*,4H, δ(NH2)), 3.7 ppm (2*,6H, δ (–N-CH3)), 2.8 ppm (2*,6H, δ( =C-CH3)), 2.16 ppm (2*, 4H, δ(CH2) . and free ligand (L) can be seen in Figure 3 showed peaks are 9.8 ppm (2*,2H, δ(OH), 7.28-7.51ppm (2*,16H, δ(CH) aromatic), 5.9 ppm (2*,4H, δ(NH2)), 3.3 ppm (2*,6H, δ (–N-CH3)), 2.3 ppm (2*,6H, δ( =C-CH3)), 2.06 ppm (2*, 4H, δ (CH2).
13C-NMR (CDCl3 , ppm , 400MHz ):for the [ZnL]Cl2 δ131(C-1), δ177(C-2), δ100(C-3), δ133 (C-4), δ119(C-5), δ159(C-6), δ69(C-7), δ189(C-8), δ60(C-9), δ66(C-10), δ144(C-11), δ133(C-12), δ158(C-13), δ120(C-14), δ122(C-15), δ112(C-16), δ166(C-17), δ67(C-18).and free ligand (L) showed peaks are δ129(C-1), δ179(C-2), δ105(C-3), δ134(C-4), δ109(C-5), δ169(C-6), δ77(C-7), δ179(C-8), δ50(C-9), δ76(C-10), δ153(C-11), δ127(C-12), δ150(C-13), δ129(C-14), δ134(C-15), δ124(C-16), δ173(C-17), δ77(C-18).The splitting pattern of the complex shows rigidity is generated in the molecule as a result of coordination. In addition, the NMR spectrum confirms the diamagnetism of zinc atom.
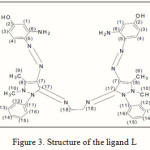 |
Figure 3: Structure of the ligand L |
Infrared spectral studies of the ligand and its complexes
The IR spectrum of the ligand shows characteristic bands at 3413, 1632, 1565 and 1035 cm-1 due to the ν(O-H), ν(C=N), ν(N=N) and ν(N-N) functional groups, respectively10. The IR spectra of the complexes exhibited ligand bands with the appropriate shifts due to complex formation (Table 2). The ν(C=N) and ν(N=N) at 1632 and 1565 cm-1 in the free ligand shift to 1620–1647 and 1528-1535 cm-1, respectively for the complexes. The reduction in bond order, upon complexation, can be attributed to delocalization of metal electron density (t2g) to the π-system of the ligand. These shifts confirm the coordination of the ligand via the nitrogens of azomethine and the azo groups to metal ions. The ν(N–H) band at 3392-3343 cm-1in the free ligand was shifted to lower frequency and by ca. 12 cm-1 for the complexes, confirm the involvement of the amine group in complexation. At lower frequency the complexes exhibited bands around 412-471 cm-1 assigned to the ν(M-N)11-15.
Table 2: IR Frequencies (cm-1) of the compounds
| Compound | ν(N-H) | ν(O-H) | ν(N=N) | ν(C=N) | ν(N-N) | ν(M-N) |
| I | 3390-3340 | 3435 | 1612 | 1034 | – | |
| L | 3392-3343 | 3413 | 1565 | 1632 | 1035 | – |
| [Co L] Cl2 | 3389-3336 | 3415 | 1535 | 1617 | 1032 | 412-470 |
| [Ni L] Cl2 | 3385-3332 | 3408 | 1528 | 1602 | 1034 | 435-425 |
| [Cu L] Cl2 | 3379-3333 | 3412 | 1531 | 1611 | 1036 | 438-478 |
| [Zn L] Cl2 | 3386-3337 | 3411 | 1533 | 1615 | 1034 | 459-471 |
Electronic spectral, magnetic moments, and conductivity measurements
The electronic spectrum of the Schiff base ligand exhibits intense absorption at (56179.7 and 25839.7) cm-1 attributed to p ®p* and n®p*, respectively. The magnetic moment value 4.42B.M of Co(II) complex is typical for a distorted octahedral geometry16. The electronic spectrum for this complex showed two broad peaks at 15822.7 and 19841.2 cm-1, assigned to 4T1g→4A2g (ν2) and 4T1g (F)→4T1g(P) (ν3) transition, respectively17,18. The Ni(II) complex exhibits peaks at 17793.5 and 14931.2 cm-1 which assign to 3A2g→3T1g(P) and 3A2g→3T1g(F) transition, respectively suggesting an octahedral geometry around the Ni(II) ion19,20. The magnetic moment value of this complex is consistent with octahedral geometry structure. The spectrum of Cu(II) complex of together with the µeff value (Table 3) suggest octahedral geometry around Cu(II)complex. The spectrum of Zn(II) complex gave band assigned to CT21. The molar conductivity values of the complexes were consistent with 2:1 electrolytes22.
Table 3: Magnetic Moments, molar Conductance values and U.V-Vis spectra data in DMF solutions.
|
Compound |
λmax nm |
Wave number (cm-1) |
εmax (L.mol-1.cm-1) |
Assignments |
Molar conductivity (ohm-1 cm2 mol-1) |
μeff (BM) |
|
L |
278 |
56179.7 |
278000 |
π→π* |
– |
– |
|
387 |
25839.7 |
387000 |
n→π* |
|||
|
[Co L] Cl2 |
632 |
15822.7 |
632000 |
4T1g→4A2g (ν2) |
158 |
4.42 |
|
504 |
19841.2 |
504000 |
4T1g (F)→4T1g (P) (ν3) |
|||
|
[Ni L] Cl2 |
562 |
17793.5 |
562000 |
3A2g→3T1g (P) |
133 |
2.82 |
|
671 |
14931.2 |
671000 |
3A2g→3T1g (F) |
|||
|
[Cu L] Cl2 |
552 |
18115.9 |
552000 |
2B1g→2Eg |
149 |
1.82 |
|
647 |
15455.9 |
647000 |
2B1g→2B2g |
|||
|
[Zn L] Cl2 |
480 |
20833 |
480000 |
C.T |
144 |
diamagnetic |
Optimized Geometries Energy and Vibrational for ligand and their Metal Complexes
A theoretically probable structure of metal complexes with azo Schiff base as shown in Figure 4 have been calculated to search the most probable model building stable structure, these shapes shows the calculated optima geometries for ligand and its metal complexes. The result of PM3 method of calculated in gas phase for the heat of formation and binding energies of ligand were tabulated in (Table 5).The vibrational spectra of the free ligand have been calculated as shown in Figure 5. The theoretically calculated wave number for the ligand showed that some deviations from the experimental values, these deviations are generally acceptable in theoretical calculations. The most diagnostic calculations vibrational where chosen for the assignment of ligand, which is included in (Table 6).
Electrostatic Potential (E. P)
Electron distribution governs the electrostic potential of molecules. The electrostatic potential (E.P) describes the interaction of energy of the molecular system with a positive point charge, so it is useful for finding sites of reaction in a molecule positive charged species tend to attack a molecule where the E.P. is strongly negative electrophilic attack23. The (E.P.) of free ligand was calculated and plotted as 3D contour to investigate the reactive sites of the molecules. The results of calculation showed that the LUMO of transition metal ion prefer to react with the HOMO of nitrogen atoms of ligand as shown in Figure 6.
Table 5: Conformation energetic in (K J.Mol-1) for the ligand and its metal complexes.
|
Conformation |
PM3 |
|
|
∆Hf ◦ |
∆Eb |
|
|
L |
163.1849634 |
-9756.4610366 |
|
[Co L]Cl2 |
-2203.431760 |
-33076.785900 |
|
[Ni L]Cl2 |
-1794.411076 |
-30871.675843 |
|
[Cu L]Cl2 |
-10978.015750 |
-29603.089765 |
|
[Zn L]Cl2 |
-1342.689754 |
-27088.829662 |
Table 6: Comparison of experimental and theoretical vibration frequencies for L.
|
Symb. |
H2L |
|
u(NH) |
(3400,3350)*(3486,3610)**(-2.5,-7.7)*** |
|
u(C= N)iso |
(1635)*(1647)**(-0.7)*** (1635)*(1630)**(0.3)*** |
|
u(OH) |
(3406)*(3501)**(-2.7)*** |
|
u(N-N) |
(1034)*(1055)**(-2.0)*** (1034)*(1022)**(1.1)** |
|
u(N=N) |
(1562)*(1552)**(0.6)*** |
*:Experimental frequency, **:Theoretical frequency,***:Error % due to main difference in the experimental measurements and theoretical treatments of vibration spectrum
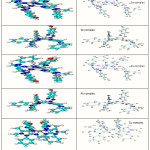 |
Figure 4: Conformational Structure of ligand and their Complexes |
 |
Figure 5: The calculated vibrational frequencies of ligand L |
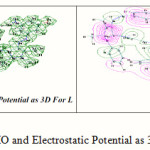 |
Figure 6: HOMO and Electrostatic Potential as 3D Contours for L |
Anti bacterial Activates
The data of antimicrobial activates of the prepared ligand and its complexes are given in (Table 6), the result showed that the complexes have more toxicity against the bacterial species than the free ligand. This can be attributed to the tweeds chelation theory24, according to which the chelation reduces the polarity of the metal atom mainly because of the partial sharing of its positive charge with donor group and possible electro delocalization over the whole ring. This increases the lipophilic character lipid layers of the cell membranes. Furthermore, the mode of action of compounds may involve the (C=N) and (N=N) groups with active centers of cell constituents resulting in the interference with normal cell process.
Table 6:Antibacterial activities for Ligand and its complexes
|
Compounds |
Staphylococcus aureus(+) |
Escherichia coli (-) |
||
|
5mM |
10mM |
5mM |
10mM |
|
|
L |
– |
– |
+ |
– |
|
[Co L]Cl2 |
– |
+ |
– |
++ |
|
[Ni L]Cl2 |
++ |
++ |
+ |
++ |
|
[Cu L]Cl2 |
++ |
+ |
+ |
+ |
|
[Zn L]Cl2 |
+ |
+ |
+ |
+ |
(-) = No inhibition = inactive,(+) = (3-7) mm = active,(++) = (7-12) mm = highly active
Conclusion
In this paper we have explored the synthesis and coordination chemistry of some monomeric complexes obtained from the reaction of the hexadentate ligand L with some metal ions as shown in Figure 7. The mode of bonding and overall structure of the complexes were determined through physico-chemical and spectroscopic methods. Complex formation study via molar ratio has been investigated and results were consistent to those found in the solid complexes with a ratio of (M:L) as (1:1).Hyper Chem-6 program has been used to predict structural geometries of compounds in gas phase, and Biological activity studies of the ligand and its metal complexes against several organisms, Escherichia coli and Staphylococcus aureus are reported, Compounds exhibited the high effect of activity.
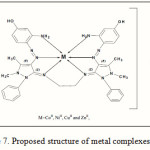 |
Figure 7: Proposed structure of metalcomplexes |
Reference
- Emeleus L. C., Cupertino D. C., Harris S. G., Owens S., Parsons S., Swart R. W., Tasker P. A., and White D. J, J. Chem. Soc., Dalton Trans., 1239 (2001).
- Nivorozhkin A. L., Toftlund H., Nivorzhkin L. E., Kamenetskaya I. A., Antsyshkina A. S., and Porai-Koshits M. A., Trans. Met. Chem., 19, 319 (1994).
- Whitener, G. D. and Hagadam J. R., J. Chem Soc. Dalton Trans., 1249 (1999).
- Patel M. M. and Patel K. C., J. Indian Chem Soc., 74, 1 (1997).
- Patel V., Patel M. and Patel R., J. Serb .Chem.Soc., 76, 727-734 (2000).
- Wengnack N. L., Hoard H.M. and Rusnak F., J. Am. Chem., 121, 9748 (1999).
- Maurya M. R., Coord. Chem. Rev., 237, 163 (2003).
- Foresman J. and Frish C, Exploring Chemistry with Electronic Structure Methods, 2nd ed ,Gaussian Inc, Pittsburgh, PA, (1996).
- Atlas M. R., Alfres E., Brown P and Lawrence C, Laboratory Manual Experimental Microbiology, Mosby, Inc., (1995).
- Raman N., Johnson S. and Raja A., Journal of Coordination Chemistry, 62, 691-709 (2009).
- Shayma S., Yang F. and Abbas A. S., European Journal of Scientific Research, 33, 4, 702-709 (2009).
- Nakamoto K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley-Inter Science, New York, (1997).
- Cross, A. D. and Alan J., An Introduction To Practical Infrared Spectroscopy, Butterworths, London, (1969).
- Dyer R. J., Application of Absorption Spectroscopy of Organic Compounds, Prentice-Hall, New Jersey, (1965).
- Socrat
- s G., Infrared Characteristic Group Frequencies, Wiely-Interscience publication, New York, (1980).
- Lever A. B. P., From Coelho to Inorganic Chemistry: A Lifetime of Reactions – By Fred Basolo, Profiles in Inorganic Chemistry, John P. Fackler Jr. (Series Ed.), Kluwer Academic Publishers/Plenum Press, New York, NY, Hardbound., Coordination Chemistry Reviews., 247,1, 197-197(1) (2003).
- Shayma A. S., Yang F., Sadia M. and Mohean E., Modern Applied Sciences, 3 (12), 88-93 (2009).
- Shayma A. S., E-Journal of Chemistry, 8 (1), 153-158 (2011).
- Shayma A. S., Yang F., Sadia M. and Mohean E., ARPN Journal of Engineering and Applied Sciences, 4 (9), 29-33 (2009).
- Shayma A. S., E-Journal of Chemistry, 7 (4), 1598-1604 (2010).
- Shayma A. S., Yang F., Sadia M. and Mohean E., Sains Malaysiana, 39 (6), 957-962 (2010).
- Temel T., Cukir U., Tolan B., Otludil, H. and Vgras V., J. Coord .Chem., 57, 7, 571 (2004).
- hamberlain B. M., SunY., Hagadorn J. R., Hemmesch E. W., Hillmyer A.V. and Tolman W. B. Macrollecules, 32, 2400 (1999).
- Morad F. M., Elajaily M., Ben Gweirif M. S., Preparation, Physical Characterization and Antibacterial Activity of Ni (II) Schiff Base Complex, 1.1, 72-78 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









