Synthesis, Characterization and Antimicrobial Screening of Cobalt(II), Nickel(II) and Copper(II) Complexes with Schiff Base Derived from 2-Phenyl Quinoxaline Thiosemicarbazone
Bimal Kumar1, B.K. Rai* and Nisha Ambastha2
1Deparment of Chemistry, L.N.T. College, Muzaffarpur, B. R. A. Bihar University, Muzaffarpur (India).
2Deparment of Chemistry, R.S.S. College, Chochahan, Muzaffarpur (India).
3C/o Vishesh Chandra Ambastha, Flat No.201, Vishal Villa Apartment, R. K. Avenue Road, R. Nagar, Patna (India).
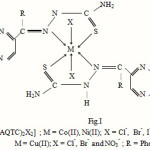
Complexes of general molecular formula [M(AQTC)2X2], where M = cobalt(II), nickel(II) and copper(II), AQTC = 2 phenyl quinoxaline thiosemicarbazone, X = Cl–, Br-, I– and NO3– have been synthesized. Complexes were characterised by analytical analyses, IR spectra, electronic spectra, molar mass, conductivity measurements. The compound AQTC acts as a neutral, bidentate ligand and bonded to the metal ion through azomethine N and sulphur atom of thiosemicarbazone moiety. The remaining coordinating positions are satisfied by anions such as Cl–, Br–, I– and NO3–. Electronic spectral and magnetic susceptibility values reveals octahedral geometry for the complexes. The complexes were found to be non electrolytic in nature on the basis of low value of molar conductance. The ligand as well as metal complexes have been screened for their antibacterial and antifungal activity.
KEYWORDS:AQTC;l cobalt(II); nickel(II); copper(II); Schiff base; antimicrobial; antifungal activity
Download this article as:| Copy the following to cite this article: Kumar B, Rai B. K, Ambastha N. Synthesis, Characterization and Antimicrobial Screening of Cobalt(II), Nickel(II) and Copper(II) Complexes with Schiff Base Derived from 2-Phenyl Quinoxaline Thiosemicarbazone. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Kumar B, Rai B. K, Ambastha N. Synthesis, Characterization and Antimicrobial Screening of Cobalt(II), Nickel(II) and Copper(II) Complexes with Schiff Base Derived from 2-Phenyl Quinoxaline Thiosemicarbazone. Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24596 |
Introduction
Schiff base ligands and their transition metal complexes have been extensively studied over past few decades1-3. Schiff base and their metal complexes are very popular due to their diverse chelating ability4. They play important role in both synthetic and structural research, because of their preparative accessibility and structural diversity5. In addition to variable magnetic property and catalytic activity, the Schiff base complexes can also serve as efficient model for the metal containing sites in metallo-proteins and enzymes6,7. In continuation8-13 of earlier work on Schiff base complexes and their biocidal activities, preparation and characterization of cobalt(II), nickel(II) and copper(II) complexes with 2-phenyl quinoxaline thiosemicarbazone are reported in this paper.
Experimental
Material and methods
All the chemicals used were of AnalaR grade. The complexes were analysed using standard procedures14. IR spectra were recorded on Perkin Elmer-577 spectrophotometer using KBr disc. The electronic spectra of the complexes were recorded on a Cary 2390 spectrophotometer. Magnetic susceptibilities were measured using Gouy balance using Hg[Co(NCS)4] as a calibrant. The molar conductance values were done on Systronics conductivity meter using DMF as a solvent.
Analytical, colour, molar mass, magnetic susceptibility, molar conductivity, electronic, spectral data and decomposition temperature are recorded in Table-1 and salient features of IR spectral data are recorded in Table-2.
Table 1: Analytical, Colour, Molar Mass, Magnetic Susceptibility, Electronic Spectra And Conductivity Measurement Data For Ligand Aqtc And Its Metal Complexes
| Compounds/ Colors | Molar Mass | % Analysis found (Calculated)M C N H | meff B.M. | lmax electronic cm-1 | Wm ohm-1 cm2 mol-1 | Decomp-osition Temp. oC | |||
| AQTCColorless | 308.00 | 62.42 (62.33) | 22.81 (22.72) | 4.61 (4.54) | |||||
| [Co(AQTC)2Cl2]Brown | 745.93 | 7.81 (7.90) | 51.58 (51.47) | 18.83 (18.76) | 3.69 (3.75) | 5.04 | 10200, 14800, 21500 | 3.1 | 272 |
| [Co(AQTC)2Br2]Brown | 839.74 | 6.93 (7.01) | 45.85 (45.72) | 16.59 (16.67) | 3.26 (3.33) | 5.07 | 10410, 14940, 21680 | 3.03 | 278 |
| [Co(AQTC)2I2]Brown | 928.74 | 6.29 (6.34) | 41.26 (41.34) | 14.93 (15.07) | 2.96 (3.01) | 4.89 | 10240, 14980, 21800 | 3.02 | 269 |
| [Co(AQTC)2(NO3)2]Brown | 798.93 | 7.28 (7.37) | 48.20 (48.06) | 17.61 (17.52) | 3.46 (3.50) | 4.99 | 10360, 15020, 21860 | 3.00 | 273 |
| [Ni(AQTC)2Cl2]Greenish yellow | 745.71 | 7.82 (7.87) | 51.57 (51.49) | 18.81 (18.77) | 3.79 (3.75) | 3.19 | 12600, 17300, 23400 | 4.8 | 263 |
| [Ni(AQTC)2Br2]Greenish yellow | 834.52 | 7.09 (7.03) | 45.86 (46.01) | 16.68 (16.77) | 3.27 (3.33) | 3.16 | 12800, 17870, 23200 | 4.7 | 288 |
| [Ni(AQTC)2I2]Greenish yellow | 928.52 | 6.39 (6.32) | 41.22 (41.35) | 15.11 (15.07) | 2.97 (3.01) | 3.12 | 12800, 18140, 23600 | 4.3 | 286 |
| [Ni(AQTC)2(NO3)2]Greenish yellow | 798.51 | 7.26 (7.35) | 47.91 (48.08) | 17.62 (17.53) | 3.55 (3.50) | 3.24 | 12840, 18240, 23250 | 4.6 | 301 |
| [Cu(AQTC)2Cl2]Green | 750.54 | 8.52 (8.46) | 51.28 (51.16) | 18.72 (18.65) | 3.67 (3.73) | 1.87 | 13600, 16800 | 2.7 | 281 |
| [Cu(AQTC)2Br2]Green | 839.35 | 7.50 (7.57) | 45.59 (45.74) | 16.78 (16.67) | 2.28 (2.35) | 1.86 | 13500, 16600 | 2.8 | 290 |
| [Cu(AQTC)2(NO3)2]Green | 803.54 | 7.82 (7.90) | 47.69 (47.78) | 17.53 (17.42) | 3.43 (3.48) | 1.93 | 13400, 16300 | 2.9 | 292 |
Preparation of the ligand
2-phenyl quinoxaline was prepared by modifying the earlier reported method15. Ethanolic solution of 2-phenyl quinoxaline (0.01 M) was treated with thiosemicarbazide hydrochloride (0.01 M) dissolved in ethanolic solution of sodium acetate in tetrahydro furan. The resulting mixture were heated on water bath for 3-4 h with occasional stirring. The precipitate was washed with water, treated with dilute sodium carbonate solution and filtered. The solid was washed thoroughly with water and recrystallized with tetrahydrofuran to furnish 2-phenyl quinoxaline thiosemicarbazone as colourless compound. m.p. 129±1oC, yield 60-65%.
Preparation of the complexes
The complexes of cobalt(II), nickel(II) and copper(II) have been prepared by reacting an ethanolic solution of the ligands AQTC and ethanolic solution of corresponding metal salts in molar ratio 2:1. The resulting mixtures were heated on waterbath for 2-3 h when the compounds separated out which were filtered, washed with ethanol followed by diethyl ether and dried in an electric oven. Yield in all cases 60-70%.
Results And Discussion
Infrared spectra
The IR spectrum of the ligands AQTC shows a broad band of medium intensity at 3460 cm-1 which can be assigned16,17 to nN-H vibrations. In the spectra of the complexes this band remains unaffected, indicating non involvement of either primary amino or secondary amino group in coordination with metal ion. The IR spectrum of the ligand shows a broad band of medium intensity at 1460 cm-1 assigned16,18 nC=N. In the spectra of the complexes this band shows red shift with silghtly reduced intensity. The shift of the band and change in intensity suggest coordination of the azomethine N. The linkage of metal ion with azomethine N is further supported by the appearance of a far ir band, in the region 475-455 cm-1 in the complexes assigned19 to nM-N. The other ir band of structural significance in the spectra of the ligand appears ~800 cm-1 assigned16,20 to nC=S. In the spectra of the complexes this band shows red shift indicating coordination through thione S atom of thiosemicarbazone moiety. The linkage through S atom wass further supported by appearance of a new band in the far IR region at 425-395 cm-1 assigned16,21 to nM-S. The evidence of metal halogen linkage are indicated by the appearance of bands in the far IR regions at 320-265 cm-1 assigned to nM–X (X = Cl–, Br–or I– ) The evidence of metal halogen is confirmed by the low value of molar conductance of the complexes in the range 2.8- 4.9 ohm-1 cm2 mol-1 (Table-1) which indicate non electrolytic nature22 of the complexes. The evidence of monocoordinate linkage of nitrate ion with metal ions are supported23,24 by the appearance of a significant IR band at 1320 cm-1 and 1200 cm-1 with a separation of 120 cm-1.
Table 2: Infrared Spectral Bands Of Ligand Aqtc And Their Metal Complexes
| Compounds | nC=N | nC= | nC=S | nM–N | nM–S | nM–X |
| AQTC | 3460 m,b | 1460 m,b | 800 s,b | |||
| [Co(AQTC)2Cl2] | 3460 m,b | 1440 m,b | 775 s,b | 455 m | 395 m | 310 m |
| [Co(AQTC)2Br2] | 3460 m,b | 1405 m,b | 770 m,b | 460 m | 410 m | 290 m |
| [Co(AQTC)2I2] | 3460 m,b | 1435 m,b | 770 m,b | 460 m | 400 m | 275 m |
| [Co(AQTC)2(NO3)2] | 3460 m,b | 1440 m,b | 775 m,b | 465 m | 405 m | |
| [Ni(AQTC)2Cl2] | 3460 m,b | 1440 m,b | 770 m,b | 470 m | 410 m | 320 m |
| [Ni(AQTC)2Br2] | 3460 m,b | 1435 m,b | 770 m,b | 465 m | 415 m | 295 m |
| [Ni(AQTC)2I2] | 3460 m,b | 1435 m,b | 775 m,b | 465 m | 410 m | 265 m |
| [Ni(AQTC)2(NO3)2] | 3460 m,b | 1440 m,b | 775 m,b | 460 m | 415 m | |
| [Cu(AQTC)2Cl2] | 3460 m,b | 1440 m,b | 775 m,b | 465 m | 400 m | 315 m |
| [Cu(AQTC)2Br2] | 3460 m,b | 1440 m,b | 770 m,b | 465 m | 400 m | 310 m |
| [Cu(AQTC)2(NO3)2] | 3460 m,b | 1435 m,b | 775 m,b | 460 m | 410 m |
On the basis of above IR spectral bands assignments, it is proposed that the compound AQTC behaves as neutral bidentate ligand and coordination proposed through azomethine N and thione sulphur of thiosemicarbazone moiety. The remaining coordinating positions are occupied by anions such as Cl–, Br–, I– and NO3–.
Electronic spectra and magnetic susceptibility of the complexes
Electronic spectra of the complexes of Cobalt(II), Nickel(II) and Copper(II) with ligand AQTC were recorded in the region 10,000-25000 cm-1. The complexes of cobalt(II) display three bands in the regions, 10660-10200, 15400-14800, 21860-21400 cm-1 which is assigned to 4T2g(F) ¬ 4T1g(F), 4A2g(F) ¬ 4T1g(F) and 4T1g(P) ¬ 4T1g(F) transitions respectively which proposes octahedral25 geometry for the cobalt(II) complexes. The proposed octahedral geometry of the Cobalt(II) complexes are further supported24,25 by high magnetic moment value in the range 4.89 – 5.07 BM. The electronic spectra of all the Nickel(II) complexes displays three bands in regions, 13000-12600, 18280-17300, 23560-23200 cm-1 which may be assigned to 3T2g(F) ¬ 3A2g(F), 3T1g(F) ¬ 3A2g(F), 3T1g(P) ¬ 3A2g(F) transitions respectively, which proposed octahedral25,30 geometry for all the Nickel(II) complexes. The proposed octahedral geometry for all the Nickel(II) complexes are further supported27,28,31 by magnetic moment value in the range 3.10-3.23 BM. The complexes of Copper(II) exhibits two spectral bands in the regions 13600-13200 cm-1 and 16800-16260 cm-1 assigned to 2T2g ¬ 2Eg and charge transfer bands respectively which support distorted octahedral25,32 geometry. The proposed geometry of copper(II) complexes are further supported27,28,33 by the magnetic moment value in the range 1.87 – 1.94 BM.
 |
Scheme 1 Click here to View scheme |
Antimicrobial activity
Antimicrobial and antifungal activity of ligand AQTC and their cobalt(II), nickel(II) and copper(II) complexes have been tested by disc diffusion technique34 at concentration level of 2.0 and 0.2% (w/v medium were used). The ligand AQTC and its metal complexes were screened for their antimicrobial activity against Gram negative bacteria, Escherichia coli. The antifungal activity were screened against, Aspergillus flavus and Aspergillus niger. Filter paper discs of diameter 12 mm were used and the diameters of zones of inhibition formed around each disc after incubating for a period of 72 h at 25-30oC. Results were compared with known antibiotics tetracycline at the same concentration. It is observed that majority of compounds show moderate activity against different strains of bacteria and fungi. On comparison with reference to ligand complexes of cobalt(II), nickel(II) and copper(II) are more effective than ligand AQTC. Better activities of some of the metal complexes as compared to the ligand can be explained by chelation theory35. The theory explains that decrease in polarizability of the metal could enhance the liphophilicity of the complexes which leads to the breakdown to permeability of the cells resulting in interference with normal cell process36.
Conclusion
Thus on the basis of above studies it may be concluded that the complexes possess the octahedral geometry around the central metal ion as shown in Fig.1.
Acknowledgement
The author is grateful to Dr. D. C. Baluni, Prof. and Head, Postgraduate Deptt. of Zoology, R. D. S. College, Muzaffarpur for his help in measurements of antimicrobial aand antifungal activities of ligand and complexes. One of the author is grateful to the University Grant Commission for financial supports under Minor Research Project.
Refrences
- A. Mitra, T. Banerjee, P. Roychoudhary, S. Choudhary, P. Bera and N. Saha, Polyhedron, 16, 3735 (1997).
- P. Bera, R. J. Butcher and N. Saha, Chemistry Letters, 559 (1998).
- P. Bera, N. Saha, S. Kumar, D. Banerjee and R. Bhattacharya, Transition Met. Chem., 24, 425 (1999).
- E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker and L. Deng, J. Am. Chem. Soc., 34, 113 (7063).
- P. Espinet, M. A. Esteruelas, L. A. Oro, J. L. Serrano and E. Sola, Coord, Chem. Rev., 117, 215 (1992).
- S. Y. Zhou, W. L. Yan and C. Z. Guang, Anal Sci., 20, 1127 (2004).
- J. Liu, H. Zhang, C. Chem., H. Deng, T. Lu and L. Ji, Dalton Trans, 119, 114 (2003).
- B. K. Rai, and Kaushlendra Sharma, Orient J. Chem., 22, 645 (2006).
- B. K. Rai, Pramod Choudhary, Upendra Prasad Singh, Poonam Sahi, Zahid Hussain and Swaty Rana, Orient J. Chem., 23, 271, 291 (2007).
- B. K. Rai, Irena Kostova, S. P. Ojha, Rashmi Tomar and V. K. Rastogi, Asian J. Phys. 16, 29 (2007); B. K. Rai, Asian J. Phys., 16, 71 (2007); B. K. Rai, H. C. Rai, Shiv Pujan Singh, Rashmi Tomar and Om Prakash, Asian J. Phys., 16, 76 (2007).
- B. K. Rai and Kaushlendra Sharma, Asian J. Chem., 20, 137 (2008); B. K. Rai, Rajeshwar Rai, Poonam Sahi and Swaty Rana, Asian J. Chem., 20, 143, 149 (2008).
- B. K. Rai, Zahid Hussain, U. P. Singh, S. N. Prasad, Anukul Prasad and P. M. Mishra, Ultra J. Chem., 4, 53 (2008); B. K. Rai and S. N. Prasad, Ultra J. Chem., 4, 71 (2008); B. K. Rai and Arvind Kumar, Ultra J. Chem., 4, 179 (2008); B. K. Rai, J. Ind. Council Chem, 25, 137 (2008).
- B. K. Rai , Ravishankar and S. Pandey, Asian J. Chem; 21, 5409, 5994 (2009); B. K. Rai and Vinayak, Ultra J Chem, 5, 67 (2009); B. K. Rai, A. Kumar and Ravishankar, Ultra J. Chem., 5, 73 (2009); B. K. Rai , S. Kumari , R. K. Singh, A Prasad, M. P Sinha and P. M. Mishra, Ultra J. Chem; 5, 83 (2009); B. K. Rai, Anukul Prasad, Vinayak, Arvind Kumar and S. Jha ‘Sunit’, Asian J. Phys., 18, 63 (2009); B. K. Rai Vineeta Singh, Vinayak, S. P. Singh and S. Jha ‘Sunit’, Asian J. Phys., 18, 67 (2009); Anukul Prasad and B. K. Rai, Orient J. chem., 25, 175 (2009); B. K. Rai, A Baluni, A. Prasad, R. Thakur, P. Prakash, 21, 3708, 3713 (2009); B. K. Rai, J. Ind. Council Chem, 26, 121 (2009); B. K. Rai, Asian J. Chem., 22, 2761 (2010); B. K. Rai and C. Kumar, Asian J. Chem., 22, 5613 (1020); B. K. Rai and S. Singh, Asian J. Chem., 22, 5619 (2010); B. K. Rai and K. K. Sharma, Asian J. Chem., 22, 5625 (2010).
- A. I. Vogel, Textbook of Quantitative Chemical Analysis, Revised by J. Mendham, R. C. Denny, J. D. Barnes and M. Thomas, Pearson Education, 7th Edn, London (2008).
- B. C. Chen, R. Zhao, M. S. Bednarz, B. Wang, J. E. Sundeen and J. C. Barrish, J. Org. Chem., 69, 977 (2004).
- R. M. Silverstein and F. X. Webster, Spectrometric Identification of Organic Compounds, 6th Edn. John Wiley and Sons, 109 (2008).
- D. Cook, Can. J. Chem., 29, 1961 (2009).
- K. B. Gudasi, S. A. Patil, R. S. Vadavi, R. V. Shenoy and M. S. Patil, J. Serb Chem. Soc., 71 (5), 529 (2006).
- D. C. Dash, R. K. Behera, (Ms) M. Sen and F. M. Meher, J. Indian Chem., Soc., 71, 693 (1994).
- R. K. Agarwal, Himanshu Agarwal and Indranil Chakraborti, Synth. React. Inorg. Met. Org. Chem., 25, 671 (1995).
- H. Kaur and S. K. Sangal, J. Indian Chem., Soc., 71, 621 (1994).
- D. A. Boghaei and N. Lashani Zadegan, Synth. React Inorg. Met. Org. Chem., 30, 1393 (2000).
- C. C. Addison, N. L. Logan, S. C. Wallwork and D. C. Barner, Quart Rev., (1971).
- R. A. NyQuist, C. L. Putzig, M. A. Leugers, Infrared and Raman Spectral Atlas of Inorganic Compounds and Organic Salts, Acadmic Press, New York (1995).
- A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier, New York (1968).
- P. S. Mane, S. G. Shirodhar, B. R. Arbad and T. K. Chondekar, Indian J. Chem., 40A, 648 (2000).
- R. L. Carlin and A. J. Van Dryneveledt, Magnetic properties of transition metal compounds, Springer-Verlag, New York (1997).
- B. N. Figgis, Introduction to ligand Field, Wiley Eastern Ltd., New Delhi, 279 (1976).
- C. J. Ballhauson and H. B. Grag, Inorg., 1, 111 (1962).
- A. K. Tahir, H. S. Shivajni, J. Nafees and K. Shoukat, Indian J. Chem, 39A, 450 (2009).
- V. K. Jetty, L. Singh and M. Shukla, Rehman and S. N. Rastogi, J. Indian Chem., Soc., 67, 987 (1990),
- A. P. Mishra, M. Khare and S. K. Gautam, Synth. React Org. Org. Chem., 32, 1485 (2002).
- N. K. Singh and N. K. Agarwal, Indian J. Chem, 37A, 276 (1998).
- P. K. Mukherjee, K. Saha, S. N. Giri, M. Pal and B. P. Saha, Indian J. Microbiology, 35, 327 (1995).
- N. Nishant, S. Ahmad and R. T. Ahmad, J. Appl. Polym. Sci., 100, 928 (2006).
- C. H. Colins and P. M. Lyne, “Microbiological Methods“, 4th ed., Butterworths, London, Boston, 235 (1976).

This work is licensed under a Creative Commons Attribution 4.0 International License.









