Design of Porous Alginate Conduit using a Freeze Drying Technique for Neural Engineering
Esmaeil Biazar
Department of Chemistry, Tonekabon Branch, Islamic Azad University, Tonekabon (Iran).
Corresponding author E-mail: e.biazar@tonekaboniau.ac.ir
Peripheral nerve injuries can lead to lifetime loss of function and permanent disfigurement. Different methods, such as conventional allograft procedures and use of biologic tubes present problems when used for damaged peripheral nerve reconstruction. Designed scaffolds comprised of natural and synthetic materials are now widely used in the reconstruction of damaged tissues. Porous alginate conduits were fabricated using a freeze drying technique and were investigated by SEM analysis. The results demonstrate that the alginate conduits have high interconnection and porosity, so they hold promise for use as neural tissue engineering scaffolds.
KEYWORDS:Nerve conduits; Tissue engineering; Alginate; Ffreeze drying
Download this article as:| Copy the following to cite this article: Biazar E. Design of Porous Alginate Conduit using a Freeze Drying Technique for Neural Engineering. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Biazar E. Design of Porous Alginate Conduit using a Freeze Drying Technique for Neural Engineering. Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24475 |
Introduction
Peripheral nerve injuries most commonly result from blunt trauma or from penetrating missiles such as bullets or other objects, but are also associated with fractures and fracture dislocations. Therefore, crush injuries are more common than nerve transections. When nerve endings are unable to be rejoined without tension, a bridging section of nerve is used and two end-to-end sutures are performed. The crushed section of the nerve is cut, removed, and replaced by a nerve taken from another (less important) site, typically the sural nerve at the back of the leg [1-6].
Since the beginning of the 1990s, there have been several published studies reporting that natural biopolymers is satisfactory as a biomaterial for regeneration of various tissues and/or organs and that it is also useful for peripheral nerve regeneration.
Alginate is one of the polysaccharides that formed hydrogel with to multiple cations such as Cu2 +, Ca2 + or Al3 + , alginate is a biocompatible material and suitable for tissue engineering especially nerve regeneration [7-9].
Prang et al assessed the capacity of alginate gels to promote directed axonal regrowth in the injured mammalian central nervous system. Multivalent copper ions were used to create the alginate-based gels, diffusion of which into the sodium alginate layers created hexagonally-structured anisotropic capillary gels. After precipitation, the entire gel was traversed by longitudinally-oriented capillaries. The alginate scaffolds promoted adult peripheral nerve survival and highly oriented axon regeneration.30 This was the first instance of using alginates to produce anisotropic-structured capillary gels [10-12].
Further studies are needed to investigate the long-term physical stability of the alginate scaffolds, because central nervous system axon regeneration can take many months to occur. However, in addition to being able to provide longterm support, the scaffolds must also be degradable. Of all the biologic and synthetic biopolymers investigated by Prang, only agarose-based gels were able to be compared with the linear regeneration achieved by alginate scaffolds [13].
In this study , porous alginate conduits designed by freeze drying method and investigated by microscopic analysis.
Materials and Methods
Alginic acid sodium salt (Merck) was used. The sodium alginate was dissolved in deionized water, which was purified by ultrafiltrationat a concentration of 2% and subsequently filtered through a 0.2 mm pore size filter. Sixty millimeters of the sodium alginate solution were placed in a cylindrical aluminum mold (5 cm in diameter) and carefully superimposed with 20 ml of a 1 mol/l copper nitrate solution using pump spray bottles (VWR). The filled mold was covered with a vaulted glass bowl and the gel was allowed to form for 24 h at room temperature avoiding concussion of the mold. gel slices were rinsed in a hydrochloric acid solution (1 mol/l) several times to exchange copper ions for protons. Gel bodies were mounted in a tube. Then tube was freeze dried at determined temperature for 24 h. The freeze dried tubes were then dried at room temperature and analyzed by Scanning electron microscopy .
Results
Figure 1 shows alginate hydrogel. The hydrogel is blue color due to presence of copper nitrate solution. The hydrogel tubes formed by mold. Figures 2 show alginate tubes that removed copper ions by hydrochloric acid solution and freeze dried.
 |
Figure 1: The hydrogel formed by mold with to copper nitrate solution
|
 |
Figure 2:The alginate tubes formed by mold and freeze dried |
Figure 3 shows microscopic images of freeze dried alginate hydrogel. Figure 3a and 3b show cross section of freeze dried hydrogel and Figure 3c and 3d show long section of freeze dried hydrogel. Result showed high porosity of samples also pores well interconnected together. Pore size mean was about 4-5 micrometer.
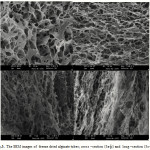 |
Figure 3: The SEM images of freeze dried alginate tubes; cross –section (3a-b) and long –section (3c-d). |
Conclusion
This paper described a method to fabricate porous alginate nerve conduits with high porosity and pore interconnection using a freeze drying technique. These conduits contained interconnected channels for guiding the outgrowing nerve fibres, an tube for preventing ingrowth of fibrous tissue into the nerve gap and allowing fluid transport. Furthermore, the porous morphology of the alginate scaffold is suitable for neural regeneration . In addition, the fabrication technique can be applied to a variety of polymers.
References
- Turgut M, Geuna S. International symposium on peripheral nerve repair and regeneration and 2nd club Brunelli meeting. J Brachial Plex Periph Nerve Inj. 2010;5:5.
- Evans GR. Peripheral nerve injury: A review and approach to tissue engineered constructs. Anat Rec. 2001;263:396–404.
- Ghaemmaghami F, Behnamfar F, Saberi H. Immediate grafting of transected obturator nerve during radical hysterectomy. Int J Surg. 2009;7:168–169.
- Firouzi M, Moshayedi P, Saberi H, et al. Transplantation of Schwann cells to subarachnoid space induces repair in contused rat spinal cord. Neurosci Lett. 2006;402:66–70.
- Bain JR. Peripheral nerve allografting: Review of the literature with relevance to composite tissue transplantation. Transplant Proc. 1998; 30(6):2762–2767.
- Godard CW, de Ruiter MD, Spinner RJ, et al. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 2009;1:91–105.
- Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, et al. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10:493–504.
- Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–23.
- Smetana Jr. K. Cell biology of hydrogels. Biomaterials. 1993;14: 1046–50.
- Prang P, Müller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569.
- Prang P, Müller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569.
- Treml H, Kohler HH. Coupling of diffusion and reaction in the process of capillary formation in alginate gel. Chem Phys. 2000;252: 199–208.
- Shoichet MS, Li RH, White ML, Winn SR. Stability of hydrogels used in cell encapsulation: an in vitro comparison of alginate and agarose. Biotechnol Bioeng. 1996;50:374–81.

This work is licensed under a Creative Commons Attribution 4.0 International License.









