Convenient Reduction of Nitro Compounds to their Corresponding Amines with Promotion of NaBH4/Ni(OAc)2.4H2O System in Wet CH3CN
Davood Setamdideh*, Behrooz Khezri and Manouchehr Mollapour
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135 - 443 (Iran).
NaBH4 in the presence of catalytic amounts of Ni(OAc)2.4H2O reduces varieties of nitro compounds to their corresponding amines. Reduction reactions were carried out in a mixture of CH3CN and H2O (3.0:0.3 ml) at room temperature with high to excellent yields of products.
KEYWORDS:Amines; Ni(OAc)2.4H2O; NaBH4; Nitro compounds; Reduction
Download this article as:| Copy the following to cite this article: Setamdideh D, Khezri B, Mollapour M. Convenient Reduction of Nitro Compounds to their Corresponding Amines with Promotion of NaBH4/Ni(OAc)2.4H2O System in Wet CH3CN. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Setamdideh D, Khezri B, Mollapour M. Convenient Reduction of Nitro Compounds to their Corresponding Amines with Promotion of NaBH4/Ni(OAc)2.4H2O System in Wet CH3CN. Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24450 |
Introduction
Reduction of nitro compounds is one of the important methods for the preparation of aryl amines. Nitro compounds have traditionally been reduced by high-pressure hydrogenation1. The application of NaBH4 as a mild reducing agent has brought about fundamental changes in the reduction of functional groups in modern organic synthesis. It is known that solely sodium borohydride does not reduce nitro compounds under ordinary conditions 2. However, the reducing power of this reagent or its polymeric analogue i.e., borohydride exchange resin (BER) undergoes a drastic change toward reduction of nitro groups by the combination with transition metal halides or salts such as NaBH4/CoCl2 3,NaBH4/FeCl2 4,NaBH4/CuSO4 5, NaBH4/Co(pyridyl) 6, NaBH4/Cu(acac)2 7, borohydride exchange resin (BER); BER/ Ni(OAc)2.4H2O, CoCl2, PdCl2, Cu(OAc)2 8 are effective for the reduction of aliphatic or aromatic nitro compounds. It was reported that by the combination of transition metal halides or salts with NaBH4 in protic or aqueous solvent systems 3, 8, 10 the formation of transition metal borides which are actively catalyzes-resulting from the decomposition of borohydride-with the evolving of hydrogen gas and in conjunction with the hydride attack reduce nitro compounds to their corresponding amines. Osby et al. 9a reported that the combination of NaBH4 with catalytic quantities of NiCl2·6H2O smoothly reduces aliphatic nitro compounds to their amines at room temperature. The method is successful for reduction of aliphatic nitro compounds. Also, Jia Wei Chen et al. 8 reported that the combination of NaBH4 with BER/ Ni(OAc)2.4H2O smoothly reduces aromatic nitro compounds to their amines at room temperature. The method is successful for reduction of Aromatic nitro compounds. however Our preliminary experiments revealed that nitro compounds could reduce to corresponding amines by NaBH4/NiCl2.6H2O/CH3OH and NaBH4/Ni(OAc)2.4H2O/CH3OH, but the amount of NaBH4 and moderated yields of products (Table 1 & 2) in the best optimized reaction conditions (Table1 & 2, entry 4) are of disadvantages. On the other hand, the results of table (2) are better than table (1). We feel worthwhile to investigate one of the more highly reactive systems for reduction of nitro compounds to their corresponding amines, the literature survey could find no published example of nitro compounds reduction by this system (NaBH4/Ni(OAc)2·4H2O/CH3OH). To expand the above mentioned strategy in the reduction of nitro compounds by the NaBH4/transation metal halide system and our continuous efforts to develop modified borohydride agents in organic synthesis 10, here we report that the combination of Ni(OAc)2.4H2O as a more efficient promoter for rapid and convenient reduction of nitro compounds with sodium borohydride in aqueous CH3CN at room temperature.
Results And Discussion
Our preliminary experiments showed that the reduction of nitrobenzene with 4 molar equivalents of NaBH4 and catalytic amounts of Ni(OAc)2.4H2O (0.2 mol) in CH3CN (as an efficient aprotic solvent) without deposition of any precipitate was completed within 3 h under reflux conditions (Table 3, entry 4). However, a mixture of products with a 50 % yield of aniline was obtained from the reduction. In another attempt, it is found that by adding a small amount of water to the reaction mixture, when there was an immediate deposition of a fine black precipitate, the rate of reduction was dramatically increased and the reaction was completed within 20 min at room temperature (Table 3, entry 6). In the latter case, aniline was the sole product of reduction. The optimization reactions showed that using 4 molar equivalents of NaBH4 and 0.2 molar equivalents of Ni(OAc)2.4H2O in a mixture of CH3CN:H2O (3.0:0.3 mL) are the best conditions for the complete conversion of nitrobenzene into aniline (scheme 1).
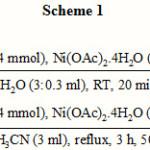 |
Scheme 1 Click here to View scheme |
We applied the optimal conditions for the reduction of a variety of nitro compounds to their corresponding amines. As shown in Table 4. The product amines were obtained in high to excellent yields within 20–60 minutes. The chemoselective reduction of nitro group in the presence of carboxylic acid was proven with the reduction of 2-nitrobenzoic acid to anthranilic acid in 94% yield (table 4, entry 4). Our attempts to reduce C=O over nitro group in 2-nitrobenzaldehyde and 3′-nitroacetophenone were unsatisfactory and under the different conditions both of the functional groups were reduced (table 4, entries 5, 6). The complete reduction of nitroarenes with two nitro groups was also achieved perfectly by this protocol: using 8 molar equivalents of NaBH4 in the presence of 0.2 molar equivalents of Ni(OAc)2.4H2O were the requirements for these transformations (table 4, entries 11, 12, 13). All attempts to perform chemoselective reduction of one nitro group in the presence of the other one were unsuccessful and the mixture of products was identified from the reaction mixture. Primary, secondary and tertiary aliphatic nitro compounds were rapidly reduced to their corresponding amines in excellent yields at room temperature (table 4, entries 15-17). The exact mechanism of this protocol is not clear; however, in our experiments, some results are noteworthy. In all reductions, by the combination of NaBH4 with Ni(OAc)2.4H2O in wet CH3CN, the immediate deposition of a fine black precipitate and the subsequent evolution of hydrogen gas were observed. We think that reduction of nitro compounds is probably due to formation of the black precipitate which catalyses the decomposition of NaBH4, strongly adsorbs nitro compounds and activates them towards reduction by NaBH4. This black precipitate may be boride, zerovalent metal or a mixture of these.
Conclusion
In conclusion we have shown that a variety of nitro compounds were reduced efficiently to their corresponding amines by the combination of NaBH4 with catalytic amounts of Ni(OAc)2.4H2O in wet CH3CN. The reductions were completed within 20–60 minutes at room temperature. We think that in the view points of molar equivalents of, NaBH4 and catalyst, high efficiency, shorter reaction times, easy work-up procedure, and presentation of Ni(OAc)2.4H2O as a more efficient catalyst for the probable boride-catalysed reduction of aromatic and aliphatic nitro compounds, this protocol is a synthetically useful addition to the present methodologies.
Experimental Section
All reagents and substrates were purchased from commercial sources with the best quality and were used without further purification. IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 300 MHz Bruker Avance spectrometers, respectively. The products were characterised by a comparison with authentic samples (melting or boiling points) and their 1H NMR or IR spectra. All yields refer to isolated pure products. TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
A Typical Procedure for Reduction of Nitrobenzene to Aniline with NaBH4/NiCl2.6H2O Ssystem
In a round-bottomed flask (15 ml) equipped with a magnetic stirrer, a solution of nitrobenzene (0.123 g, 1 mmol) in CH3CN-H2O (3:0.3 ml) was prepared. To the resulting solution, NiCl2.6H2O (0.049 g, 0.2 mmol) was added and the mixture was then stirred for 5 min. Afterwards, NaBH4 (0.151g, 4 mmol) as a fine powder was added to the reaction mixture and a fine black precipitate was immediately deposited. The mixture continued to be stirred for 20 min and the progress of the reaction was monitored by TLC (eluent; CCl4/Et2O: 5/2). At the end of reaction, distilled water (5 ml) was added to the reaction mixture and the mixture stirred for 10 min. The mixture was extracted with CH2Cl2 (3 × 8 ml) and dried over anhydrous sodium sulfate. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/3) gave the pure liquid aniline (0.085 g, 92%, entry 1, Table 4).
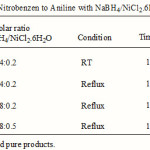 |
Table 1 Click here to View table |
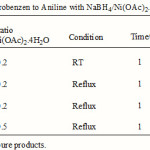 |
Table 2 Click here to View table |
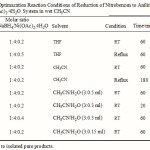 |
Table 3 Click here to View table |
 |
Table 4 Click here to View table |
Acknowledgment
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- (a) Stiles, M.; Finkbeiner, H. L. J. Am. Chem. Soc, 81: 505 (1959). (b) Finkbeiner, H. L.; Stiles, M. J. Am. Chem. Soc, 85: 616 (1963).
- (a) Burk, S. D. and Danheiser, R. L. Handbook of Reagents for Organic Synthesis, Oxidising and Reducing Agents, Wiley-VCH: New York, (1999). (b) Seyden-Penne, J. Reductions by the Alumino and Borohydrides in Organic Synthesis, Wiley-VCH: New York, (1997). (c) Hudlicky, M. Reductions in Organic Chemistry, Ellis Horwood, Chichester, (1984).
- Satoh, T.; Suzuki, S.; Miyaji, Y.; Imai, Z. Tetrahedron Lett, 10: 4555 (1960).
- Ono, A. H.; Sasaki, H.; Yaginuma, F. Chem. Ind. (London), 480 (1983).
- Yoo, S. E.; Lee, S. H. Synlett, 419 (1990).
- Hanaya, K.; Muramatsu, T.; Kudo, H.; Chow, Y. L. J. Chem. Soc. Perkin Trans. I, 2409 (1979).
- (a) Chem, J. W.; Qin, C. Q. React. Polym, 16: 287(1992). (b) Yoon, N. M.; Choi, J. Synlett, 135 (1993).
- Ganem, B.; Osbey, J. O. Chem. Rev, 86: 763(1986).
- (a) Osby, J. O.; Ganem, B. Tetrahedron Lett, 26: 6413(1985). (b) Nose, A.; Kudo, T. Chem. Pharm. Bull, 29 (1981). (c) Sarma, J. C.; Borbaruah, M.; Sharma, R. P. Tetrahedron Lett. 26: 4657 (1985). (d) Sharma, D. N.; Sharma, R. D. Tetrahedron Lett, 26: 2581 (1985).
- (a) Zeynizadeh, B.; Setamdideh, D. J. Chin. Chem. Soc, 52: 1179 (2005) (b) Setamdideh, D.; Zeynizadeh, B. Z. Naturforsch, 61b: 1275 (2006). (c) Zeynizadeh, B.; Setamdideh, D. Synth. Commun, 36: 2699 (2006). (d) Zeynizadeh, B.; Setamdideh, D. and Faraji, F. Bull. Korean Chem. Soc, 29: 76 (2008). (e) Zeynizadeh, B.; Setamdideh, D. Asian J. Chem. 21: 3588 (2009). (f) Zeynizadeh, B.; Setamdideh, D. Asian J. Chem, 21: 3603 (2009). (g) Setamdideh, D.; Khezri, B. Asian J. Chem, 22: 5566 (2010).
- Aldrich Catalogue of Fine Chemicals, (2004–2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









