Chemical Modification of α-amylase from Locale Bacteria Isolate Bacillus subtilis ITBCCB148 with Glyoxylic Acid
Yandri*, Nina Anggraini, Tati Suhartati and Sutopo Hadi
Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Lampung, Bandar Lampung 35145 (Indonesia).
Chemical modification of α-amylase from locale bacteria isolate Bacillus subtilis ITBCCB148 using glyoxylic acid to increase thermal stability of the enzyme has successfully been done. The results showed that the native and the modified enzyme have similar pH of 6.0 and optimum temperature of 60°C. However, the thermal stability of the modified enzymes was increased 1.8-2.1 times compared to the native enzyme. The thermal stability data obtained at 60°C for 60 minutes as following: the native enzyme has residual activity 17% and half-life (t1/2) = 25.3 min., while the modified enzymes with modification degree of 67, 69 and 82% have residual activity of 39, 44 and 47%, respectively and t1/2 of 46.2, 53.3 and 49.5 minutes, respectively.
KEYWORDS:α-amylase; Chemical modification; Glyoxylic acid; B. subtilis ITBCCB148
Download this article as:| Copy the following to cite this article: Yandri Y, Anggraini N, Suhartati T, Hadi S. Chemical Modification of α-amylase from Locale Bacteria Isolate Bacillus subtilis ITBCCB148 with Glyoxylic Acid. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Yandri Y, Anggraini N, Suhartati T, Hadi S. Chemical Modification of α-amylase from Locale Bacteria Isolate Bacillus subtilis ITBCCB148 with Glyoxylic Acid. Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24438 |
Introduction
α-Amylase is one of the enzymes which is widely used in industrial processes1. The use of enzyme in industrial sector requires some specific requirements such as the enzyme must be stable at higher temperature (thermostable) and it can work in extreme pH condition. However, these requirements are not fulfilled by most enzymes, as the enzyme normally work at physiological condition1. Therefore, in order to be used in industrial sector, there must be effort to increase the stability of the enzyme.
There are three known methods to increase the stability of enzyme2, i.e. immobilization process, directed mutagenesis and chemical modification. Chemical modification is one of the preferred methods to increase the stability of water-soluble enzyme as the use of enzyme immobilization has a weakness of mass transfer inhibition by immobile matrix which causes the decrease of binding activity and reactivity of the enzyme, while directed mutagenesis requires comprehensive information about the primary structure and three-dimensional structure of the enzyme. According to Janecek3, in immobilization process, the mechanism of the enzyme used in clinical treatment during interaction with receptor or other components from cellular membrane, might change due to the long matrix. In chemical modification, the interaction between enzyme and substrate is not hindered by the presence of insoluble matrix, so the decrease of enzyme activity might be suppressed.
Melik-Nubarov et al.4 reported that hydrophilization of α-chymotrypsine using glyoxylic acid with NaBH4 as reducing agent was significantly able to increase the stability of the enzyme. The modification was performed at pH 8.4, as a result the primary ammine group of the side-chain of lysine on the surface of the enzyme was easily reacted with glyoxylic acid.
Chemical modification with glyoxylic acid has also been done by Soemitro5 on α-amylase obtained from Saccharomycopsis fibuligera. The result showed that the modified enzyme has optimum pH of 5.5, optimum temperature 50°C, and the stability has increase 1.2 times compare to the native enzyme. Yandri et al.6 have also successfully modified α-amylase from B. subtilis ITBCCB148 using dimethyladipimidate (DMA).
In this paper we reported the chemical modification of α-amylase from B. subtilis ITBCCB148 using glyoxylic acid.
Experimental
Materials
All chemicals used were of high grade (pro analysis) materials. Local bacteria isolate B.subtilisITBCCB148 was obtained from Microbiology and Fermentation Technology Laboratory, Chemical Engineering Department, Bandung Institute of Technology, Bandung, Indonesia.
Research procedure
The following research phases were done: production, isolation, purification, chemical modification and characterization of the native and modified enzymes were based on our previous report7.
Activity test of a-amylase and determination of protein content
Activity of α-amylase was determined based on Fuwa method8 and using dinitrosalicylic acid reagent9. The protein content was determined based on the method by Lowry et al.10.
Chemical modification of the native enzyme using glyoxylic acid4
To 10 mL of native α-amylase (containing 0.4 μmol/mL) mixed with 0.5% amylum in buffer borate pH 8.4 was added with 10 μmol glyoxylic acid and 8 μmol NaBH4, the reaction was carried out for 30 minutes at 4°C.
Characterization of the native and modified enzymes
The characterizations of the native and modified enzymes including: determination of modification degree, determination of optimum pH and determination of thermal stability.
Determination of modification degree11
Determination of modification degree was done based on the method used by Synder and Sobocinski (1975) and as follows: 0.1 mL of native enzyme was dissolved into 0.9 mL borate buffer (pH 9.0) and then was added with 25 μl 0.03 M 2,4,6-trinitrobenzene-sulfonic acid (TNBS). The mixture was then shaken and left it at room temperature for 30 min. The standard solution was made with the same composition but using the native enzyme, while the blank solution contained 1 mL borate buffer 0.1 M pH 9 and 25 μl 0.03 M TNBS. The absorbance was measured at the λmax. 420 nm.
Determination of optimum temperature of the native and modified enzymes
The determination of optimum temperature of native and modified enzymes was done by varying the temperature at 55, 60, 65, 70, 75 and 80°C.
The stability test of the native and modified enzymes
The stability of native and modified enzymes was done based on the known procedure which entailed measuring the residual activity of the enzyme after being incubated for 0,10, 20, 30, 40, 50, and 60 minutes at optimum temperature, where the initial activity of enzyme without heating was given a value of 100%.
Determination of half-life (t½), ki and DGi
Determination of ki value (thermal inactivation rate constant) of the native enzyme and
the modified enzyme was done using the first order of inactivation kinetics equation (Eq. 1)13:
ln( Ei / E0 )= -ki t (1)
Where Ei and E0 are the activity of the inactivated and initial forms of the enzyme, respectively: ki is the inactivation rate constant of the enzyme and t is the time.
The denaturation energy change (D Gi) of the native and modified enzymes was done using Eq. 215:
D Gi = -RT ln (kih/kBT) (2)
Where ki is the inactivation rate constant of enzyme, kB is the Boltzmann constant, h is Planck’s constant and T is the absolute temperature and R is the universal gas constant.
Results and Discussions
Determination of modification degree of native and modified enzymes
The chemical modification of native enzyme with glyoxylic acid was done in three concentration variation of glyoxylic acid at 3.7, 7.4 and 14.8 mg. The result of modification degree is shown in Table 1.
Table 1: Determination of modification degree using 2,4,6-trinitrobenzene-sulfonic acid11
|
Sample |
∆A420 nm |
Modification (%) |
| Native enzyme |
0.0970 |
0 |
| Modified with Glyoxylic acid 3.7 mg |
0.0320 |
67 |
| Modified with Glyoxylic acid 7.4 mg |
0.0300 |
69 |
| Modified with Glyoxylic acid 14.8 mg |
0.0175 |
82 |
Determination of modification degree was done based on the comparison of lysine residue of modified and native enzymes. The ammine group on lysine residue which was not modified, i.e. did not bind to glyoxylic acid, would react with 2,4,6-trinitrobenzene-sulfonic acid (TNBS) molecule which produced the yellow complex. The more the ammine group on lysine residue bound to glyoxylic acid (higher modification degree), the less free ammine group reacted with TNBS. As a result, the yellow complex formed would also be less. The qualitative test was based on the yellow complex formed where the higher modification degree, the yellow complex formed was less. Table 1 illustrated the modification process with glyoxylic acid to the native enzyme produced modification degree of 67, 69 and 82%, respectively. These data informed us that the higher the concentration of glyoxylic acid added, the higher the modification degree obtained.
Determination of optimum pH of the native and modified enzyme
The results of characterization showed that the native enzyme and the modified enzyme in all modification degree have the same optimum pH of 6.0 as shown in Figure 1.
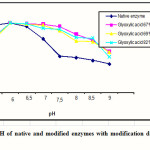 |
Figure 1: Optimum pH of native and modified enzymes with modification degree of 67,69 and 82% Click here to View figure |
Figure 1 also showed that the native enzyme was stable between pH range of 5 to 7, while at pH 7.5 to 9, its activity was decreased significantly. All modified enzymes were stable in pH range of 6.0 to 9.0. This result indicated that the modified α-amylase had better working pH range compare dwith the native enzyme, especially at basic condition.
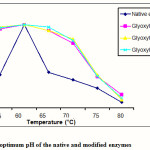 |
Figure 2: The optimum pH of the native and modified enzymes |
Determination of optimum temperature of the native and modified enzyme
The result of determination of optimum pH of the native and modified enzymes is shown in Figure 2. All modified enzymes have similar optimum pH with the native enzyme. According to Soemitro5 the increase of activity due to the increase of temperature did not change the accuracy of enzyme tertiary structure (either before or after modification) which could draw closer the side chain of some amino acids, as a result the optimum temperature of the enzyme did not change. Similar results were also observed by other researchers13,14 which stated that the chemical modification did not always cause the change of optimum temperature to the enzyme which was modified.
Figure 2 also indicated that the modified enzymes were more stable at higher temperature than the native enzyme particularly at 65 and 70°C. Furthermore, this result also informed us that the modification caused the enzyme to be more rigid and it was much more stand against temperature.
Thermal stability of the native and modified enzyme
The thermal stability of the native and modified enzymes was determined by counting the percentage of their residual activity. The residual activity was determined by incubating each enzyme at 60°C for 60 minutes and the result is shown in Figure 3.
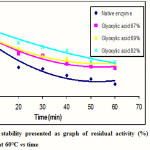 |
Figure 3: Thermal stability presented as graph of residual activity (%) of the native and modified enzymes at 60°C vs time |
Figure 3 showed the residual activity (%) of each enzyme at 60°C for 60 minutes. The native enzyme has residual activity of 17%, while the modified enzymes with modification degree of 67, 69, and 82% have residual activity of 39, 44, and 47%, respectively. Based on the above graph, it was clearly shown that the modified enzymes have thermal stability much higher than the native enzyme. It was also noted that the higher the modification degree used, the higher the thermal stability of the modified enzyme.
Chemical modification α-amylase with glyoxylic acid affected significantly toward the structure stability of the modified enzyme. The mechanism of structure stability of the enzyme by glyoxylic acid occurred due to the formation of cross-linking via inter- or intramolecule of glyoxylic acid to form small spheres of enzyme and to protect the unfolding of enzyme tertiary structure. According to Kazan et al.15, the higher the modification degree, the more the hydrogen boding formed, consequently the enzyme rigidity would also be higher. The result observed in this work was also similar where the higher the modifying agent used, the higher the thermal stability of the modified enzyme obtained.
Rate of thermal inactivation (ki), half-life (t1/2) and denaturation energy change (ΔGi) of native and modified enzymes
The values of rate of thermal inactivation (ki), half-life (t½) and denaturation energy change (ΔGi) of the native and modified enzymes with glyoxylic acid are shown in Table 2.
Table 2: The values of rate of thermal inactivation (ki), half-life (t½) and denaturation energy change (ΔGi) of the native and modified enzymes ( 67, 68 and 82%)
| Enzyme |
ki (min.-1) |
t1/2 (min.) |
∆Gi (Kj mol-1) |
| Native enzyme |
0. 027 |
25.3 |
103.18 |
| Modified with Glyoxylic acid 3.7 mg |
0.014 |
46.2 |
104.83 |
| Modified with Glyoxylic acid 7.4 mg |
0.013 |
53.3 |
105.18 |
| Modified with Glyoxylic acid 14.8 mg |
0.013 |
49.5 |
105.06 |
The half-life of the modified enzymes was increased between 1.8-2.1 times compared to the native enzyme. The results obtained in this work are almost similar to result previously reported by other5on chemical modification of α-amylase from S. fibuligera which was also modified using glyoxylic acid. Stahl16 stated that the half-life of the enzyme determined the enzyme stability. As can be seen from Table 2, all half-life of the modified enzymes were increased quite significant.
The decrease of ki value of all modified enzymes indicated that the rate of denaturation was decreased. Based on Table 2, the ki value of all modified enzymes was decreased about twice compared to the native enzyme. The less ki value indicated that the enzyme is less flexible in water due to the bond formation between glyoxylic acid and NH2 group on the side chain of lysine residue present on the surface of the enzyme, as a result the unfolding of enzyme will also be decreased and the enzyme structure will be more rigid and become more stable. As shown in Table 2, the energy change due to the denaturation (∆Gi) of all modified enzymes were also increased which indicated that these enzyme became more rigid and much less flexible in water.
Conclusions
Based on the results discussed above it was clearly observed that the chemical modification with glyoxylic acid was able to increase the thermal stability of the native α-amylase up to 1.8-21 times. The data obtained based on the observation of the decrease of ki value, the increase of half-life and energy denaturation change, and it was also found that all modified enzymes were more stable than the native enzyme.
Acknowledgments
The authors would like to thank to The Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of National Education of Republic of Indonesia that provides fund for this project to be undertaken through competency based research scheme (Hibah Kompetensi) with contract number of 364/SP2H/PL/Dit. Litabmas/IV/2011, 14 April 2011.
References
- Vieille C. and Zeikus J.G., Thermozymes: Identifying molecular determinant of protein structural and functional stability, Tibtech., 14 (6), 183-189 (1996).
- Mozhaev V.V., Siksnis V.A., Melik-Nubarov N.S., Galkantaite N.Z., Denis G.J., Butkus E.P., Zaslavsky B.Y., Mestechkina N.M. and Martinek K., Enzyme Stabilization Via Hydrophilization. Covalent Modification of Trypsin and α-chymotrypsin. Eur. J. Biochem. 173:147-154 (1987).
- Janecek S., Strategies for obtaining stabel enzymes. Proc. Biochem., 28 : 435-445 (1993).
- Melik-Nubarov, N.S., Mozheav, V.V., Siksnis, V.A. and Martinek, K., Enzyme Stabilization of α-chymotrypsin by Reductive Alkylation with Glyoxylic Acid. Biotechnol. Lett. , 9:725-730 (1987).
- Soemitro, S., The Effect of Selective Chemical Modification towards Stability of α-amylase from Saccharomycopsis fibuligera. Bionatura , 7: 259-273 (2005) (Indonesian)
- Yandri, Apriyanti, Suhartati T., Hadi S., The increase of thermal stability of α-amylase from locale bacteria Isolate Bacillus subtilis ITBCCB148 by chemical modification with dimethyladipimidate, Biosci. Biotechnol. Res. Asia, 7(2): 713-718 (2010).
- Yandri, Suhartati T. and Hadi S., Purification and Characterization of Extracellular a-Amilase Enzyme from Locale Bacteria Isolate Bacillus subtilis ITBCCB148. Eur. J. Sci. Res., 39: 64-74 (2010).
- Fuwa H., A new method for microdetermination of amylase activity by the use of amylase as the substrate. J. Biochem. (Tokyo), 41: 583-603 (1954).
- Mandels M., Raymond A. and Charles R., Measurment of saccharifying cellulose. Biotech & Bioeng. Symp. No 6. John Wiley & Sons Inc (1976).
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J., Protein meansurement with the folin phenol reagen, J. Biol. Chem., 193-269 (1951).
- Synder S.L. and Sobocinski P.Z., An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines, Anal. Biochem., 64: 284-288 (1975).\
- Yang Z., Michael D., Robert A., Fang X.Y. and Alan J.R., Polyethylene glycol-induced stabilization of subtilisin, Enzyme Microb. Technol., 18: 82-89 (1996).
- Kazan D., Ertan H. and Erarslan A., Stabilization of Escherichia coli Penicillin G acylase agains thermal inactivation by cross-linking with dextran dialdehyde polymers, Appl. Microbiol. Biotechnol. 48 : 191-197(1997).
- Francis G.E., Delgado C. and Fisher, D., PEG Modified Protein in Stability of Protein Pharmaceutical Part B, Ahern, T. J and M. C. Manning Editor. Manning Editor, Plenum Press, New York. Pp. 246-247(1992).
- Kazan D., Ertan H. and Erarslan, A., Stabilization of Penicillin G Acylase Against pH by Chemical Cross-Linking. Proc. Biochem., 31: 135-140 (1996).
- Stahl S., Thermophilic Microorganisms: The Biological Background for Thermophily and Thermoresistance of Enzymes in Thermostability of Enzymes (Gupta, M.N. editor), Springer Verlag, New Delhi, 59-60 (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









