Ultrasonic and Viscometric Studies of Some Amino Acids in the Aqueous Solution of Alcohols
P. Kumar, S. Kumar, S. Singh* and R. S. Gangwar
Department of Chemistry, Ganjdundwara (P. G.) College, Ganjdundwara India.
Ultrasonic & Viscometric studies of glycine and other amino acids have been carried out in methanol + water and propanol-1 + water co-solvents at different temperatures 30, 35 and 40°C respectively. Various thermodynamic parameters such as apparent molal compressibility and their limiting values have been determined. The results are discussed in the terms of solute – solvent interactions in alcohol water co-solvent. These studies establish that the amino acids have strong water structure making and breaking properties.
KEYWORDS:Ultrasonic; viscometery; aminoacids and alcohols
Download this article as:| Copy the following to cite this article: Kumar P, Kumar S, Singh S, Gangwar R. S. Ultrasonic and Viscometric Studies of Some Amino Acids in the Aqueous Solution of Alcohols. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Kumar P, Kumar S, Singh S, Gangwar R. S. Ultrasonic and Viscometric Studies of Some Amino Acids in the Aqueous Solution of Alcohols. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=25033 |
Introduction
Amino acids are the organic compounds containing both amino and carboxylic group in their molecular solutions. Amino acid in aqueous solution are ionized and can act as acids or bases due to formation of zwitter ion. The knowledge of the acid base properties of amino acids is extremely important in under standing many properties of proteins1 besides, the biological system consists of 70% water and hence the study of interaction of these amino acids has become significant. These amino acids are very important because these are the building blocks of proteins which are very essential for us.
The study of Ultrasonic velocity leads to a better understanding of the nature of interactions between the solute and solvent. In recent years there has been an increasing interest in study of non electrolytic components in aqueous and non aqueous solution2-4. The physical properties of dilute aqueous solutions of non electrolytes depends solute is a water structure breaker or maker. The influence of small quantity of amino acids on the hydrogen bonded structure of water in the solution of water, rich water methanol and water propanol mixture is quite different from that in the absence of amino acids in order to study the nature of molecular interactions in the above solutions. Ultrasonic velocity, density & viscosity studies were carried out in aqueous solutions of methanol and propanol-1 containing glycine.
Experimental
Ultrasonic velocity was measured with ‘Ultrasonic interferometer (Mittal Enterprises)’ at 2 MHz. The measuring cell in made up stainless steel which have special double walled provision for circulating water at any desired temperature by electronic thermostate controlled both to maintain the experimental liquid at a constant temp. during the experiment the chemicals were used of AR (BHD) quality. Solutions of different concentrations were made using double distilled water and alcohol.
Density measurements were reformed with a precalibrated, bicapillary pyknometer. The accuracy of the density measurements was up to ±0.000lg Cm-3. The viscosity of different samples were measured by suspended level Canon unbbehnlahde type viscometer. Uncertainty in the measured viscosities was ±0.005 cp. The ultrasonic parameters such as molar sound velocity, apparent molal compressibility, isentropic compressibility. Specific viscosity were computed in these solutions at all the concentrations using the described standard formulas5.
Result and Discussion
The variation of ultrasonic velocity with the concentration of Glycine in the solution (50% methanol +50% water) and 50% propanol-1 +50% water is tabulated in the Table-1 it can be seen from table-1, that ultrasonic velocity of solutions increases with increase in the concentration of electrolyte in the solution of Glycine + methanol + water and as well as in the solution of Glycine + propanal-1 + water at different temperatures. The values of ultrasound velocity for same sample decreases on increasing temperature similar trend of variations of velocity observed for other amino acids. These values are also agreed with the results of A Srinivasa Rao6
Table 1: Ultrasound velocity of Glycine in Methanol + Water and Propanol-1 + water (m/sec) at 30, 35 & 40°C ± 0.05°C.
|
Glycine +Methanol + Water |
Glycine + Propanol-1 + water |
|||||
| Con. (mol/lit) | 30°C | 35°C | 40°C | 30°C | 35°C | 40°C |
| 0.02 | 1513 | 1508 | 1503 | 1466 | 1463 | 1459 |
| 0.04 | 1516 | 1511 | 1506 | 1469 | 1466 | 1462 |
| 0.06 | 1519 | 1514 | 1509 | 1472 | 1469 | 1465 |
| 0.08 | 1522 | 1517 | 1512 | 1475 | 1472 | 1468 |
| 0.10 | 1525 | 1520 | 1515 | 1478 | 1475 | 1471 |
| 0.12 | 1528 | 1523 | 1518 | 1481 | 1478 | 1474 |
| 0.14 | 1531 | 1526 | 1521 | 1484 | 1481 | 1477 |
| 0.16 | 1534 | 1529 | 1524 | 1487 | 1484 | 1480 |
| 0.18 | 1537 | 1532 | 1527 | 1490 | 1487 | 1483 |
| 0.20 | 1540 | 1535 | 1530 | 1493 | 1489 | 1486 |
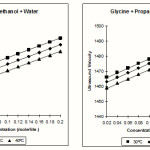 |
Figure 1 Click here to View Figure |
Table 2: Density of Glycine in Methanol + Water and Propanol-1 + water at 30, 35 & 40°C ± 0.05°C.
| Glycine +Methanol + Water | Glycine + Propanol -1+ water | ||||||
| Con. (Mol/lit) | 30°C | 35°C | 40°C | 30°C | 35°C | 40°C | |
| 0.02 | 0.9228 | 0.9142 | 0.9087 | 0.9133 | 0.9057 | 0.8982 | |
| 0.04 | 0.9235 | 0.9149 | 0.9093 | 0.9140 | 0.9063 | 0.8990 | |
| 0.06 | 0.9242 | 0.9155 | 0.9099 | 0.9197 | 0.9069 | 0.8996 | |
| 0.08 | 0.9249 | 0.9163 | 0.9105 | 0.9152 | 0.9075 | 0.9002 | |
| 0.10 | 0.9255 | 0.9169 | 0.9111 | 0.9158 | 0.9081 | 0.9008 | |
| 0.12 | 0.9262 | 0.9175 | 0.9117 | 0.9164 | 0.9087 | 0.9014 | |
| 0.14 | 0.9269 | 0.9182 | 0.9123 | 0.9170 | 0.9094 | 0.9020 | |
| 0.16 | 0.9276 | 0.9189 | 0.9129 | 0.9176 | 0.9100 | 0.9026 | |
| 0.18 | 0.9283 | 0.9196 | 0.9135 | 0.9182 | 0.9006 | 0.9032 | |
| 0.20 | 0.9290 | 0.9202 | 0.9191 | 0.9188 | 0.9112 | 0.9038 | |
Table 3: Apparent Molal Compressibiloty “fK” of Glycine in Methanol + Water and Glycine in Propanol-1+ water (cm2 / dyne x109) at 30, 35 & 40°C ± 0.05°C.
| Con. (mol/Lit) | 30°C | 35°C | 40°C | 30°C | 35°C | 40°C | |
| 0.02 | -259.47 | -253.00 | -248.32 | -238.66 | -233.72 | -228.71 | |
| 0.04 | -260.70 | -254.21 | -249.47 | -239.83 | -234.84 | -229.81 | |
| 0.06 | -261.93 | -255.39 | -250.63 | -240.96 | -236.17 | -230.90 | |
| 0.08 | -263.16 | -256.62 | -251.79 | -242.08 | -237.43 | -232.00 | |
| 0.10 | -264.37 | -257.80 | -252.69 | -2423.22 | -238.20 | -233.11 | |
| 0.12 | -265.67 | -258.99 | -254.13 | -244.37 | -239.32 | -234.21 | |
| 0.14 | -266.85 | -260.20 | -255.51 | -245.52 | -240.48 | -235.32 | |
| 0.16 | -268.10 | -261.43 | -256.47 | -246.66 | -241.61 | -236.51 | |
| 0.18 | -269.35 | -262.65 | -257.65 | -247.82 | -242.75 | -237.55 | |
| 0.20 | -270.60 | -263.84 | -258.83 | -248.98 | -243.88 | -238.66 |
Table : 4 Viscosity “η” of Glycine & Methanol + Water and Propanol-1 + water in (cp) at 30, 35, 40°C ± 0.05°C.
| Glycine +Methanol + Water | Glycine + Propanol + water | ||||||
| Con. (mol/lit) | 30°C | 35°C | 40°C | 30°C | 35°C | 40°C | |
| 0.02 | 1.1203 | 0.9859 | 0.8596 | 1.8243 | 1.5979 | 1.3450 | |
| 0.04 | 1.1231 | 0.9888 | 0.8621 | 1.8303 | 1.6045 | 1.3488 | |
| 0.06 | 1.1270 | 0.9918 | 0.8647 | 1.8366 | 1.6100 | 1.3557 | |
| 0.08 | 1.1304 | 0.9947 | 0.8693 | 1.8430 | 1.6153 | 1.3597 | |
| 0.10 | 1.1331 | 0.9976 | 0.8699 | 1.8494 | 1.6207 | 1.3655 | |
| 0.12 | 1.1371 | 1.0006 | 0.8724 | 1.8555 | 1.6263 | 1.3696 | |
| 0.14 | 1.1403 | 1.0035 | 0.8750 | 1.8615 | 1.6316 | 1.3740 | |
| 0.16 | 1.1437 | 1.0064 | 0.8726 | 1.8672 | 1.6372 | 1.3789 | |
| 0.18 | 1.1470 | 1.0094 | 0.8802 | 1.8732 | 1.6425 | 1.3838 | |
| 0.20 | 1.1503 | 1.0125 | 0.8828 | 1.8798 | 1.6480 | 1.3887 | |
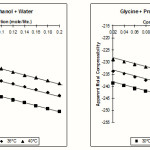 |
Figure 2 Click here to View figure |
Table : 5 Isentropic compressibility of Glycine in Methanol + Water and Propanol-1 + water (cm2 / dynex1012) at 30, 35 & 40°C ± 0.05°C.
| Con. (Mol/Lit) | 30°C | 35°C | 40°C | 30°C | 35°C | 40°C | |
| 0.02 | 47.34 | 48.10 | 48.71 | 50.95 | 51.58 | 52.29 | |
| 0.04 | 47.11 | 47.87 | 48.49 | 50.70 | 51.34 | 52.04 | |
| 0.06 | 46.89 | 47.65 | 48.26 | 50.46 | 51.09 | 51.79 | |
| 0.08 | 46.67 | 47.42 | 48.04 | 50.22 | 50.78 | 51.54 | |
| 0.10 | 46.46 | 47.20 | 47.82 | 49.99 | 50.61 | 51.3 | |
| 0.12 | 46.24 | 46.99 | 47.60 | 49.75 | 50.37 | 51.06 | |
| 0.14 | 46.3 | 46.77 | 47.38 | 49.52 | 50.13 | 51.82 | |
| 0.16 | 45.81 | 46.55 | 47.16 | 49.29 | 49.90 | 50.56 | |
| 0.18 | 45.60 | 46.33 | 46.95 | 49.05 | 49.66 | 50.34 | |
| 0.20 | 45.39 | 46.12 | 46.73 | 48.83 | 49.43 | 50.10 |
The values of density of the solution of Glycine in CH3OH + H2O and CH3 – CH2-CH2OH+ H2O tabulated in table 2 their values show that the density of solution is increases with increase in concentration of Glycine and it is decreases on increasing the temperature. Same variation is also observed for other amino acids.
The values of apparent molal Compressibility fK and viscosity are also tabulated in the Table No 3 & 4. The viscosity of solutions increases with increase in the Concentration of glycine. The variation in the value of apparent molal compressibility also agreed with Aswar & Rohankar.7
Ultrasonic velocity in the aqueous solution of CH3OH and propanol-1 increases with the addition of amino acids. When amino acids are dissolved the water structure is disturbed initially, followed by a structural reorganization leaving the molecules in closely fitting helical cavities8. This will increases the close – packed structure of water which means increased cohesion in the medium. The increase in the ultrasonic velocity in these solutions may be attributed to the cohesion brought about by the ionic hydration it is known that aqueous solutions of glycine contains in addition to the uncharged molecules NH2CH2COOH, an electrically neutral molecule, VIZ., +NH3CH2COO– dipolar ions (Zwitter ions). When the amino acids is dissolved in aqueous alcohol, the cations NH3+ and anions COO– are formed. The water molecules are attacked to the ions strongly by the electrostatic forces which introduce a greater cohesion in the solution. The cohesion in these generally increases with the increase of amino acid concentration. The increased associations observed in these solutions, may also be due to water structure enhancement brought by the increase electrostriction in the presence of methanol and propanol-1. The electrostriction effect which brings about the shrinkage in the volume of solvent, caused by the Zwitter ionic portion of the amino acid, is increased in mixed solvent as compared to that in pure water. This effect is similar to the results of sandu et al9the decrease in adiabatic Compressibility, observed in aqueous CH3OH and propanol-1 solutions with amino acids in the present study generally confirms that conclusion drawn in the velocity studies. The importance of the interactions of the water molecule in biological structures can not be overemphasized. The structures and function of biological molecules are directly linked with its aqueous medium. Water molecules constitute an integral part of the protein structure and interaction of the protein with the water molecules decides the conformation of the residues, which are on the surface of the protein molecule. The interaction has an important role in protein folding. Ordered water molecules also mediate protein-ligand interactions. Water molecules bound to active sites are found to reduce the entropy of activation when replaced by the ligands. Water bridges between carbonyl oxygen atoms and amide protons of different peptides lead to formation of linkages that stabilize the protein – ligand and protein-protein interfaces. Water molecules provide the necessary plasticity to the protein molecules which is essential for its internal dynamics and thus for its biological activity. In the molecular recognition process in enzymes and binding proteins, optimization energy of mutual hydrogen bonded networks between protein, water and ligand plays an important role. Linear results of viscosity results have also been reported for some ternary electrolytes in dioxane water mixture Das10 these results of viscosity indicate that there is a significant interactions between the solute and solvent molecules because the presence of amino and carboxylic group of Glycine, with alcohols create the possibility of interaction between the molecules.
Therefore in the light of Ultrasound Velocity and their acoustic properties. It is concluded that there is a significant interactions between solute and Solvent molecules.
References
- Lehninger, Principles of biochemistry (worth Publishers New York) 1984.
- Mishra K, Puri AK, Haroon S & Pandey J D. Indian J. Chem. 36 A (1997) 393.
- Pandey J.D. , Puri A. K. , Tiwari A & Sharma A K, Proc Indian acad Sci (Chem. Sci.) III (1999), 747.
- Wadhwani V.R. & Akhtan Y. Indian J. chem. 34 A (1995) 954.
- Riddick J A & Bunger W B, Techniques of Chemistry, Vol. II, organic solvents (wiley mInterscience, New York), 1970.
- A. Srinivasa Rao Ind. J of Chem. 37 A 659-662 (1998).
- A. S. Aswar, S. G. Kulkarni & P G Rohankar, Ind. J. of Chem. 39 A 1214-1217, 2000.
- Kavanau J, water and solute water interactions (Holden-Day Amsterdam) 1964.
- Sandu J S & Gurbir Singh, J Indian Chem. SOC, 65 (1992) 135.
- Das R. B. Ind J Chem. 15 A, 1098 (1977).

This work is licensed under a Creative Commons Attribution 4.0 International License.









