The Chemical Functionalization of Carboxylated Multi-Wall Nanotubes With 2-Aminophenole Via Microwave Irradiation
Hasan Tahermansouri1, Davood Chobfrosh Khoei2 and Masoumehmeskinfam3
1Department of Chemistry, Ayatollah Amoli Branch, Islamic Azad University, PO Box 678 Amol Iran.
2Department of Chemistry, Shabestar Branch, Islamic Azad University, Shabestar Iran.
3Department of Chemistry, Lahijan Branch, Islamic Azad University, Lahijan Iran.
In this paper, we have reported a highly efficient approach to functionalize carboxylated multi-walled carbon nanotubes (MWNT-COOH) by 2-aminophenole under microwave irradiation. Reducing the reaction time to the order of minutes and the number of steps in the reaction procedure is the major advantage of this procedure respect to conventional functionalization methods. These functionalizations involve amidation and cycloaddition reactions, respectively. The amidation was completed in one step as in the conventional methods, an additional step that is acid chloride formation was eliminated here. The cycloaddition of modified-MWNTs with phosphoryl trichloride to producing MWNT-benzoxazole was carried out in 30 min under microwave conditions, and the results were similar to what was achieved in 4 days using conventional methods. The interesting point is that modified MWNT can be homogeneously dispersed in DMF without sonication and the dispersed MWNT does not sediment in 3 month. The resulting nanotubes were characterized by FT-IR spectroscopy, Thermogravimetric analysis, Raman spectroscopy, Scanning electron microscopy and Elemental analysis.
KEYWORDS:Microwave; Functionalization; 2-aminophenole; Multi-wall nanotubes
Download this article as:| Copy the following to cite this article: Tahermansouri H, Khoei D. C, Masoumehmeskinfam M. The Chemical Functionalization of Carboxylated Multi-Wall Nanotubes With 2-Aminophenole Via Microwave Irradiation. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Tahermansouri H, Khoei D. C, Masoumehmeskinfam M. The Chemical Functionalization of Carboxylated Multi-Wall Nanotubes With 2-Aminophenole Via Microwave Irradiation. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24833 |
Introduction
Carbon nanotubes (CNTs), which were discovered by Iijima (1) in 1991, have been attracted chemists and scientists interest to their unique mechanical, thermal, and electrical properties (2-3). Since carbon nanotubes are insoluble and inert, it is often required to increase the solubility of them in organic solvents through appropriate functionalization. The chemical functionalization of CNTs involves the generation of chemical moieties on their surface that it has been well summarized in several published review articles(4-6).
So far, so many efforts by using conventional chemical methods, such as refluxing and sonication, have been applied for functionalizing CNTs but most of them are time consuming and tedious process, and require accurate control of temperature, atmosphere, and reaction time. For example, further functionalization, such as acyl chlorination and amidation (7) require additional days of reaction time. Therefore, developing techniques for rapid chemical functionalization of CNTs are necessary. It is known that Chemistry under microwave radiation is faster, and more efficient than the conventional chemical method (8-13). Some reactions of CNTs such as carboxylation (9), cycloaddition (12,14-15), electrophilic addition (16), radical (17) and amidation (12) functionalization have been done by using microwave-induced method and remarkable effect has been achieved. So, regarding to time consuming process of carbon nanotubes functionalization via conventional methods, we decided using microwave radiation for modification of MWNT-COOH. In previous paper, we have investigated the functionalization of MWNTs with 1,2-phenylenediamine (18) by refluxing. In this paper, we investigated the functionalization of MWNTs with 2-aminophenole via microwave. These modifications are two steps. These two steps involve amidation, which was completed in one step as compared to two in the conventional approach (acid chloride formation step was eliminated here), and cycloaddition reaction, respectively, with phosphoryl trichloride to produce benzoxazole on the MWNT-COOH. Synthesis route of modified MWNT-COOH is shown in Fig. 1. The products were characterized by FT-IR, Raman, SEM, TGA, DTG and Elemental analysis.
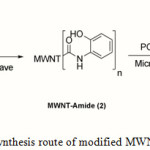 |
Figure 1:Synthesis route of modified MWNT-COOH |
Material and methods
All reagents and solvents (2-aminophenole, POCl3, THF and DMF) from Merck Chemical Inc. and MWNT-COOH (%95 purity, 20-30 nm ,Netvino Co., Ltd) were purchased and used as received.The experiments were carried out in a Ethos Model microwave oven (Milestone Co., Ltd.) with a 100 ml reaction chamber, which was lined with Teflon PFA and fitted with a 0–100 bar pressure controller. Fourier transform infra red (FTIR) spectrum was recorded using KBr tablets on a Thermo Nicolet Nexus 870FTIR spectrometer. Raman spectra we recorded on VARIAN-CARY 100 spectrometer. Scanning Electron Microscope (SEM) was used to study the morphology of the MWNTs. SEM measurement was carried out on the XL30 Philips Electron Microscope. Elemental analyses of C, H, N were performed with a SERIES (ΙΙ) 2400 from Perkin Elmer Co.USA. The samples investigated by thermal gravimetric analysis (TGA; Dupont instrument 951,USA) in the N2 (10˚C/min).
Preparation of MWNT-Amide (2).
40 mg of the MWNT-COOH was mixed with 100 mg 2-aminophenole and then were sonicated in 20 ml of DMF for 30 min. Subsequently, the mixture was loaded into an extraction vessel. The microwave power and pressure was set to 800 W and 8 bar, respectively and the reaction was carried out for 30 min. After cooling to room temperature, the mixture was filtered and washed thoroughly with DMF, ethyl alcohol and THF. Thus, the black solid was vacuum dried at room temperature for 5h.
Preparation of MWNT-benzoxazole (3).
15 mg of MWNT-Amide was mixed with 20 ml POCl3 and then were sonicated for 5 min to achieve completely dispersed solution. The suspension was irradiated by microwave (800 W) for 20 min. After, the mixture cooling to room temperature, the reaction mixture was separated by centrifugation and washed thoroughly with THF and ethyl alcohol. Thus, the obtained solid was dried under vacuum for 4 h.
Results and discussion
Elemental analyses of the modified-MWNT 1-3 are shown in Table 1. Apart from the carbon values, the atomic percentages of: H 1.13% and N 1.23% of 2 (as compared to 1) indicated that 1 is functionalized with 2-aminophenole. On the other hand, decreasing the percentage of H from 1.13 to 0.84% confirms the five-ring formation. Based on these data coupled with the assumption that the atomic percentages of nitrogen and hydrogen were originated from the employed aromatic amine without elimination, we confirmed the functionalization of MWNT-COOH with 2-aminophenole.
Table 1. Elemental analysis of the modified-MWNT 1-3.
|
MWNT |
%C |
%H |
%N |
|
1 |
96.4 |
0.04 |
0 |
|
2 |
82.6 |
1.13 |
1.23 |
|
3 |
86.6 |
0.84 |
1.07 |
The Fig. 2 presents the FT-IR spectrum of modified MWNTs. In 1, the peak at 1558 cm-1 is assigned to the active carbon stretching mode of the MWNTs-COOH (19) that forms the framework of the carbon nanotube sidewall. The appearance of the absorption peaks at 1725 and 1045 cm-1 in the IR spectra of MWNT-COOH clearly indicates carboxylic groups on MWNTs (20). The two bands at 2800-2950 cm-1 which are seen in all spectra are assigned to CH stretching of MWNT-COOH defects. In the spectrum 2, the appearance of the two new peaks at 3200-3500 cm-1 (N-H and OH stretching modes) and 1672 cm-1 indicates that 2-aminophenole has been successfully anchored onto the external surface of MWNTs. In addition, the peak at 1725 cm-1 has been almost disappeared after the 2-aminophenole treatment, whereas the peak at 1672 cm-1, which was attributed to C(=O)NH stretching vibration, has been increased remarkably. From these results it can be deduced that the formation of amide bonds can lead to the strong linkage of 2-aminophenole with carboxylic groups of MWNT-COOH. In the spectra 3 the peaks of amide group disappear and the remarkable peak at around 1625 cm-1 are appeared which can be assigned to (C=N) bond (21).
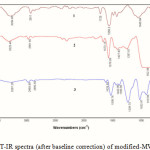 |
Figure 2: FT-IR spectra (after baseline correction) of modified-MWNTs 1-3. |
The peaks at around 1540-1580, 1400-1530, 1200-1380 and 1000-1100 cm-1 correspond to C=C stretching nanotube, aromatic ring modes, C-N and C-O stretching modes, respectively. Thus, FT-IR spectra confirm that MWNT-COOH has been successfully modified by 2-aminophenole.
Raman spectroscopy was also applied to provide structural information about MWNT-COOH before and after functionalization. As shown in Fig. 3, the D and G- bands of the MWNT ,which originate from the in- plane tangential stretching mode of carbon-carbon bonds and a disorder- induced feature owning to the presence of amorphous or disordered carbon in the MWNTs samples, respectively (22-25), at around 1356 and 1560-1585 cm-1, can be clearly observed for both MWNT-COOH and MWNT-benzoxazole. Additionally, the D- and G- bands intensity ratio (ID/IG) for MWNT-benzoxazole is 1.06, which ratio is greater than that of MWNT-COOH (0.86). This indicates a partial destruction of the conjugation structure of the MWNT sidewall because of benzoxazole attaching. In other words, the increase in intensity of the D bond at 1357 cm-1 related to the sp3 hybridization of carbon and it is used as an evidence of the disruption of the aromatic system of π electron by the attached molecules (23, 26-27).
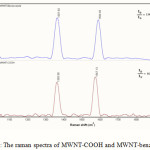 |
Figure 3: The raman spectra of MWNT-COOH and MWNT-benzoxazole |
More evidence for the MWNT-COOH, and MWNT-benzoxazole was obtained by SEM images. In Fig. 4, SEM images of MWNT-COOH and MWNT-benzoxazole are shown. In the SEM image of MWNT-COOH, it seems that the uniform surfaces of nanotubes are relatively smooth. In contrast, in the SEM image of MWNT-benzoxazole, the benzoxazole almost formed a continuous phase, and it shows that the functionalized nanotubes due to a covalently bonded 2-aminophenole on the surface of the MWNT (the rough part) become thicker. In other words, the diameters of MWNT-benzoxazole are slightly increased as compared to that of MWNT-COOH. The structure of MWNT-benzoxazole are quite different from those of the starting MWNT-COOH, in which the tube surface is relatively smooth and clean as depicted in Fig. 4.
Other evidence for the functionalization of MWNTs is thermogravimetric analysis (TGA, DTG) results that provide quantitative information on the nanotube functionalization. Since MWNT-COOH are almost thermally stable as shown in Fig. 5, the weight loss before MWNTs decomposition can be used to estimate the quantity of various groups attached to nanotube by TGA. According to Fig. 5, in TGA graph of MWNT-Amide, one decomposition below 210 ˚C is observable that can be assigned to 2-aminophenole. The TGA curve of MWNT-benzoxazole presents two decomposition regions. The first region (120–170˚C) may be corresponded to decomposition of 2-aminophenole groups (as compared with the TGA curve of MWNT-Amide) on the surface of MWNT-benzoxazole, and the second region (170–240 ˚C) should be due to decomposition of benzoxazole. If the mass loss of the MWNT-COOH at 250 ˚C (%0.57) is used as the reference, the mass loss of functionalized MWNT by 2-aminophenole and benzoxazole of MWNT-Amide and MWNT-benzoxazole at 250 ˚C is about %15 and %9.4 (for second decomposition), respectively. In latter curve, the mass loss of the first decomposition at 170˚C is about %4. This shows that there is some residual 2-aminophenole on the MWNT surface. These results indicate that there is one amide group for MWNT-Amide per 64.2 and one benzoxazole group for MWNT-benzoxazole per 94.8 carbon atoms of MWNT, respectively (18).
The DTG curve provides further evidence for covalent modification. The one major peak at 155 ˚C could be attributed to the loss of the 2-aminophenole groups bonded to MWNT. On the other hand, two peaks at 135 and 197 ˚C of MWNT-benzoxazole DTG curve can be assigned to the loss 2-aminophenole and benzoxazole, respectively.
 |
Figure 4: The SEM images of MWNT-COOH (1) and MWNT-benzoxazole(3) |
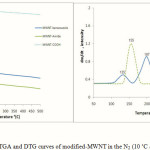 |
Figure 5: TGA and DTG curves of modified-MWNT in the N2 (10 ˚C /min) |
Dispersion test give a fair idea whether the modification on the carbon nanotubes has been achieved or not. Fig. 6 presents a photograph of two vials containing MWNT-COOH and MWNT-Benzoxazole dispersed in DMF. As it can be seen from Fig. 6, MWNT-COOH are insoluble in DMF while the modified CNTs can be directly dispersed in DMF(without sonication) homogeneously and no precipitation was found even after it was sealed for 3 months at room temperature.
 |
Figure 6: The images of MWNT-COOH(1) and MWNT-benzoxazole(3) in DMF (1mg/8ml) after standing for 3 month |
Conclusion
In summary, we have introduced benzoxazole groups onto the surface of nanotubes via reaction of MWNT-Amide with POCl3 in microwave condition. By this functionalization, MWNT solubility increased and active sites for further reactions are provided.
Acknowledgments
The financial and encouragement support provided by Research vice Presidency of Ayatollah Amoli branch, Islamic Azad University and Iranian Nanotechnology Initiative (Govt. of Iran).
References
- Iijima S. and Ichihashi T., Nature. 363, 603(1993).
- Avouris P., Acc Chem Res. 35, 1026(2002).
- Reich S., Thomsen C., Maultzsch J., Carbon Nanotubes, Wiley-VCH, 2010.
- Sun YP., Fu K., Lin Y. and Huang W., Acc Chem Res. 35,1096(2002).
- Tasis D., Tagmatarchis N., Bianco A. and Prato M., Chem. Rev. 106, 1105(2006).
- Holzinger M., Vostrowsky O., Hirsch A., Hennrich F., Kappes M., Weiss R., et al. Angew Chem Int Ed. 40, 4002(2001).
- Peng H., Alemany L., Margrave J. and Khabashesku V., J. Am. Chem. Soc. 125, 15174 (2003).
- Brunetti FG., Herrero MA., Mun˜oz JM., Dı’az-Ortiz A., Alfonsi J., Meneghetti M., Prato M. and Va’zquez E., J Am Chem Soc. 130, 8094(2008).
- Wang YB., Iqbal Z. and Mitra S., J. Am Chem Soc. 128, 95(2006).
- Wang CY., Chen TH., Chang SC., Cheng SY. and Chin TS., Adv Funct Mater. 17,1979(2007).
- Raghuveer MS., Agrawal S., Bishop N. and Ramanath G., Chem Mater. 18, 1390(2006).
- Wang YB., Iqbal Z. and Mitra S., Carbon. 43, 1015(2005).
- Bensebaa F., Zavaliche F., L’Ecuyer P., Cochrane RW. and Veres T., J Colloid Interface Sci. 277, 104(2004).
- Li JX. and Grennberg H., Chem Eur J. 12, 3869(2006).
- Brunetti FG., Herrero MA., Mun˜oz JM., Giordani S., Dı’az-Ortiz A., Filippone S., Ruaro G. Meneghetti M., Prato M. and Va’zquez E., J Am Chem Soc. 129, 14580(2007).
- Xu Y., Wang XB., Tian R., Li SQ., Wan L., Li MJ., et al., Appl Surf Sci. 254, 2431(2008).
- Liu J., Zubiri MR., Vigolo B., Dossot M., Fort Y., Ehrhardt JJ., et al, Carbon. 45, 885(2007)
- Azizian J., Tahermansouri H., Biazar E., Heidari S. and Chobfrosh Khoei D., International Journal of Nanomedicine, 5, 907(2010).
- Saini R.K., Chiang I.W., Peng H., Smalley R.E., Billups W.E., Hauge R.H. and Margrave J.L., J. Am.Chem. Soc. 125, 3617(2003).
- Mawhinney D., Naumenko V., Kuznetsova A., Yates J. and Liu J., J. Am. Chem. Soc. 122, 2383 (2000).
- Wan L., Wang X., Wang S., Li S., Li Q., Tian R. and Li M.,. J. Phys. Org. Chem. 22, 331(2009).
- Dyke C. and Tour J., Chem Eur J. 10, 812(2004).
- Gabriel G., Sauthier G., Fraxedas J., Moreno-Man M., Martinez M. T., Miravitlles C. and Casabo´J., Carbon. 44, 1891(2006).
- Zhang L., Kiny V.U., Peng H., Zhu J., Lobo R.F.M., Margrave J.L., et al., Chem Mater. 16, 2055(2004).
- Pan H., Liu L., Guo Z.X., Dai L., Zhang F., Zhu D., et al., Nano Lett. 3, 29(2003).
- Banerjee S., Hemraj-Benny T. and Wong S.S., Adv. Mater. 17, 17(2005).
- Mitchell CA., Bahr JL., Arepalli S., Tour JM. and Krishnamoorti R., 35, 8825(2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









