Synthesis, Characterization and Antimicrobial Properties of Titanium(III) complexes with Schiff Bases Derived From -Amino Acids
Trapti Dixit, P.N. Saxena And Shamim Ahmad
Department of Chemistry Bareilly College, Bareilly - 243 001, India.
The complexes of Ti(III) with the ligands glycine indole-3-aldehyde(GIA),glycine 2,4-dihydroxy- benzaldehyde (GDHB),L-alanine indole-3-aldehyde (AIA), L-alanine 2,4-dihydroxybenzaldehyde (ADHB) and valine 2,4-dihydroxy benzaldehyde (VAIA) have been synthesised and characterised by analytical, conductivity,magnetic, infrared and electronic spectral data.Based on analytical data 1:2 (M:L) stoichiometry has been suggested. The electronic spectra and magnetic data suggested octahedral geometry for all complexes. The ligands and their respective Ti(III) complexes have been screened for their antimicrobial activities.
KEYWORDS:
Ti(III); ligand,octahedral; antimicrobial activity.
Download this article as:| Copy the following to cite this article: Dixit T, Saxena P. N, Ahmad S. Synthesis, Characterization and Antimicrobial Properties of Titanium(III) complexes with Schiff Bases Derived From -Amino Acids. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Dixit T, Saxena P. N, Ahmad S. Synthesis, Characterization and Antimicrobial Properties of Titanium(III) complexes with Schiff Bases Derived From -Amino Acids. Available from: http://www.orientjchem.org/?p=24924 |
Introduction
Schiff bases are important class of ligands and have got-wide applications in various fields 1,2. The metal complexes derived from schiff bases have been found to have potential application in industry and biology3. The derivatives of such compounds as such and also after suitable structural modifications may become potential candidates for industry. In the absence of any complicating donor side chains, the naturally occuring amino acids have been found to coordinate solely through the amino and carboxylate groups forming stable chelate rings with the metal ions 4-6. First row transition metal complexes of virtually all the amino acids have been studied in considerable detail but these are virtually few reports regarding Ti(III) complexes. Keeping this fact in view, we have studied Ti(III) complexes.
Experimental
All the chemicals used in present work, various aldehydes and amino acids were of AR grade or equivalent purity. Ti(III)chloride was prepared in the lab by the standard method given in chemical literature. The ligands as well as metal complexes were analysed by standard methods. Melting points were determined by open capillary method and are uncorrected. Conductivity measurements were carried out on Philips conductivity Bridge model PR 9500 using 10-3 M (25oc) DMSO & DMF solutions at Deptt. of Chemistry, Bareilly College, Bareilly. The IR spectra were recorded using Perkin Elmer Pc-16f FTIR spectrophotometer by using KBr pellets. Magnetic susceptibility was determined by Gouy’s balance using CuSO4.5H2O as calibrant. Electronic spectra were recorded by Beckmann DU spectro-photometer .
Preparation of Ligands
NaOH(10m mol,0.4g) was dissolved in methanol (30ml) and the amino acid(10m mol) was added to it. The mixture was stirred magnetically at room temperature(20oC). When the mixture became homogeneous, a solution of the aldehyde(10m mol.) in ethanol (20 ml) was added. After 2 minutes the solution was evaporated to 20% of its’s original volume and 1ml of CH3COOH was added immediately. After 2 hours crystals appeared. Which were purified by recrystallisation. The purity of the samples was checked by TLC.
Preparation of Metal Complexes
An ethanolic solution of the ligand (2.5m mol) was added to a solution of the titanium (III) chloride (1.25 m mol) in a similar solvent at 40 oc. The mixture was stirred and boiled for 5 min. The precipitation was induced by the addition of acetonitrile. The micro crystalline complexes, so obtained, were filtered, washed successively with ethanol and ether and dried in vacuum.The entire procedure was carried out under the atmosphere dry nitrogen gas in a glove bag in order to avoid the possible oxidation of Ti (III).
Results and Discussion
The analytical data of the compounds are given in table-1.
Table 1: CHARACTERISATION OF COMPLEXES PREPARED
| Magnetic | MOLAR CONDUCTANCE
Values (ohm-1cm2 mol1) |
||||||||||
| S.NO. | N A M E OF C O M P L E X | MOLECULAR | M.Pt. | ELEMENTAL ANALYSES | Moment | ||||||
| FORMULA | (In 0C) | %Metal | % C | % H | % N | %Cl | (B.M.) | DMSO | DMF | ||
| 1 | L-Alanine 2,4 di hydroxy benzaldehyde titanium (III) chloride | [Ti(C10H10NO4)2]Cl | 3100 | 9.60 | 48.06 | 4.00 | 5.61 | 7.10 | 1.69 | 50 | 78 |
| (9.51) | (48.04) | (3.90) | (5.50) | (7.40) | |||||||
| 2 | L-Alanine Indole -3- aldehyde titanium (III) chloride | [Ti(C12H11N2 O2)2.2H2O]Cl | 2900 | 8.72 | 52.42 | 4.73 | 10.20 | 6.46 | 1.71 | 60 | 76 |
| (8.65) | (52.35) | (3.86) | (10.00) | (6.41) | |||||||
| 3 | Glycine 2,4 di hydroxy benzaldehyde titanium (III) Chloride | [Ti (C9H8NO4)2]Cl | 2700 | 10.16 | 45.82 | 3.4 | 5.94 | 7.53 | 1.72 | 54 | 68 |
| (10.12) | (45.75) | (3.35) | (5.90) | (7.45) | |||||||
| 4 | Glycine Indole -3- aldehyde titanium (III) chloride | [Ti(C11H9N2O2)2.2H2O]Cl | 2860 | 9.18 | 50.63 | 4.22 | 10.74 | 6.81 | 1.75 | 59 | 75 |
| (9.15) | (50.60) | (4.20) | (10.70) | (6.75) | |||||||
| 5 | Valine 2,4 di hydroxy benzaldehyde titanium (III) Chloride | [Ti(C12H14NO4)2]Cl | 2970 | 8.62 | 51.85 | 5.04 | 5.04 | 6.40 | 1.74 | 67 | 72 |
| (8.58) | (51.80) | (5.01) | (5.00) | (6.00) | |||||||
The structural determination of synthesized Schiff bases was done on the basis of their I.R., 1H-NMR, 13C-NMR and CHN analytical data.
The appearance of new bands in the range of 1620-1640 cm-1. in the I.R. spectra of the ligands assignable to Schiff base azomethine linkage confirm the formation of the ligands. The 1H- NMR and 13C- NMR spectral data was assigned by comparing the chemical shifts of these molecules to those of known similar structures.
The comparison of IR spectra of the ligands (GIA),(GDHB),(AIA),(ADHB) and (VAIA) with their respective Ti(III) complexes revealed important information about various coordination sites. The comparison also revealed monobasic bidentate nature for (GIA) & (AIA) and monobasic tridentate nature for (GDHB), (ADHB) and (VAIA).
The band in the range of 1640-1620 cm-1 due to (C=N) in the IR spectra of all the ligands showed a downward shift in their corresponding Ti(III) complexes. These shifts suggested the coordination through nitrogen atom of azomethine group7. This was further supported by appearance of non ligand band in the IR spectra of Ti(III) complexes in the range of 520-550cm-1, assignable to (Ti-N).
In the IR spectra of the ligands the (COO-) is greater than 200 cm-1 which [(COO-)asym at 1600cm-1 and (COO-)sym at 1400cm-1] indicates the unidenticity of the carboxylate group7. The asymmetric stretching asym(COO-) has shifted to higher frequency and the symmetric carboxyl stretching sym(COO-) has shifted to lower frequency in the IR spectra of all the Ti(III) complexes.These shifts indicated coordination between metal ion and carboxylate oxygen atom8.
In the case of the ligands (GDHB),(ADHB) AND (VAIA) a band has appeared in the region 1535-1520 cm-1 which has been assigned to (C-O) (phenolic) stretching9. These bands have shifted to higher frequency in the IR spectra of the metal complexes, suggesting coordination of the phenolic oxygen with metal ion. It was further supported by the appearance of non-ligand band in the far IR spectra of the complexes in 440-400 cm-1 region attributable to (M-O) vibrations10.
In the complexes of Ti(III) derived from the ligands (GIA) and (AIA),the coordinated nature of water molecules has been indicated by the appearance of new bands in their respective IR spectra in the region of 3400-3200 cm-1 due to (OH) vibrations of coordinated water and OH rocking and wagging bands in the regions 800-750 cm-1 and 720-700 cm-1 respectively11.
The electronic spectra of the complexes exhibited only one band in the range of 19607-20410 cm-1,which has been assigned to 2T2g2Eg transition, characteristic of octahedral geometry12. The µeff value of 1.69-1.74 B.M. for all the complexes indicated the paramagnetic nature with one unpaired electron, thereby suggesting octahedral geometry13. Conductivity of the complexes was measured in DMSO & DMF and all the complexes were found to be 1:1 electrolytic in nature of the type[ML]+Cl-. The aforesaid physico chemical evidences suggested octahedral geometry for all Ti(III) complexes.
All the ligands and their respective Ti(III) complexes were screened for their antibacterial activity . A series of glass tubes14,15 containing different concentrations of the synthesized compounds in DMF,with Muller-Hinton broth was inoculated with the required amount of inoculum to obtain a suspension of microorganism which contained 105 colony forming units per millilitre.One growth control tube was prepared without the addition of compound and one blank tube was inoculated at 37 oc for 24 h .The turbidity produced was recorded by using a uv-visible spectrometer.The minimum inhibitory concentration (MIC-mg L-1 ) was considered to be the lowest concentration which exhibited the same turbidity as the blank tube.The results are tabulated below.
Table 2: AntimicrobiolActivity MIC (mgL-1)
| S. No. | Micro- organism | COMPOUND | ||||||||||||||||||
| GTA | GDHB | AIA | ADHB | VAIA | [Ti(GIA)]cl | [Ti(GDHB)]cl | [Ti(AIA)]cl | [Ti(ADHB)]cl | [Ti(VAIA)]cl | Sulphaguanidine | ||||||||||
| 1 | S.aureus | 225 | 220 | 150 | 210 | 170 | 275 | 245 | 210 | 250 | 190 | 1200 | ||||||||
| 2 | B.pumulis | 210 | 190 | 140 | 210 | 160 | 245 | 225 | 165 | 240 | 180 | 1400 | ||||||||
| 3 | B.subtilis | 200 | 180 | 150 | 190 | 150 | 230 | 210 | 170 | 220 | 180 | 1200 | ||||||||
| 4 | E.coli | 200 | 210 | 160 | 190 | 160 | 220 | 240 | 180 | 200 | 170 | 1500 | ||||||||
| 5 | S.abony | 210 | 200 | 160 | 190 | 170 | 220 | 200 | 190 | 210 | 190 | 1500 | ||||||||
| 6 | K.pneumoniae | 250 | 250 | 250 | 225 | 250 | 250 | 300 | 325 | 310 | 290 | 1700 | ||||||||
These results revealed that the metal complexes are more potent antibacterial as compared to corresponding ligands .
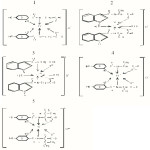 |
Figure 1 Click here to View figure |
References
- H.C.Freeman, Adv protein Chem.22,257(1967)
- M.N.Hughes,The Inorganic chemistry of Biological Process( John Wiley, New York),63(1972).
- Mahesh K. Singh,Samhita Bhaumik and Ram A. Lal, J. Indian Chem. Soc.,84,418(2007).
- Mohammad S Ameerunisha Begum, Sounik Saha, Manirathinam Nethaji and Akhil R chakravarty, Indian Journal of chemistry 48A, 473,(2009).
- Jagdish Prasad,Prem Yadav, Ved Prakash and Krishna Srivastava, J.Indian chem.Soc.,84,647,(2007).
- B.V.Mrudula Rao,G Venkatanarayana and P.Lingaiah Indian Journal of chemistry, 27A,261(1988).
- Mohammad S Ameerunisha Begum, Sounik Saha, Munirathinam Nethaji and Akhil R Chakravarty, Indian Journal of chemistry 48A, 473,(2009).
- P.K. Sharma, A.K. Sen and S.N.Dubey, Indian Journal of chemistry 33, 1031-1033(1994).
- Mahesh K singh, Samhita Bhaumika and Ram A. Lal, J . Indian Chem. Soc., 84, 418 (2007).
- N.Saha and D. Bhattacharya, Indian J Chem,21A, 574(1982).
- Meenakshi V.Patil and Sheela P. Salve, J. Indian Chem. Soc.,81,683(2004).
- S. R. Aswale, P. R. Mandlik, S. S. Aswale and A. S. Aswar, Indian Journal of Chemistry 42A, 322 (2003).
- Anant Prakash and shamim Ahmad, oriental journal of chemistry, 25(2),391(2009)
- Hussain M.M., Islam N, Khan R, Islam Md, Bangladesh J.pharmacol.2, 66-70 (2007)
- Hawkey P.M., Lewis D.A., Medical Bacteriology: A Practical approach, Oxford university Press, Oxford. 181-194(1994)

This work is licensed under a Creative Commons Attribution 4.0 International License.









