Synthesis, Characterisation and Spectral Studies of Metal Complexes of Glimepiride, An Oral Antidiabetic Drug
S.A. Iqbal, Sibi Jose and Suman Malik
1Department of Chemistry, Saifia Science College, Bhopal - 462 001, India.
2Research Laboratory, Department of Chemistry Sadhu Vaswani College.
Metal complexes of manganese (II) and cobalt (II) have been synthesized with active hypoglycemic agent (glimepiride), an oral antidiabetic drug. The elemental analyses of the ligand as well as metal complexes indicate that complexes having 2:1 stoichiometry of the type (C24H34N4O5 S)2 M.2H2O, where M= Mn(II) and Co(II). The complexes have been characterized by IR spectra, electronic spectral and molar conductance data. Infrared spectral studies confirm the co-ordination of sulphonyl oxygen on one side and enolic oxygen attached from other side with the metal ion. On the basis of electronic spectral values the complexes are proposed to have octahedral geometry. The molar conductivity also reveals that the complexes are non-ionic in nature.
KEYWORDS:Glimepiride; Synthesis; Characterization; Metal Complexes; Infrared spectroscopy.
Download this article as:| Copy the following to cite this article: Iqbal S. A, Jose S, Jacob G. Synthesis, Characterisation and Spectral Studies of Metal Complexes of Glimepiride, An Oral Antidiabetic Drug. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Iqbal S. A, Jose S, Jacob G. Synthesis, Characterisation and Spectral Studies of Metal Complexes of Glimepiride, An Oral Antidiabetic Drug. Available from: http://www.orientjchem.org/?p=24760 |
Introduction
Glibenclamide (Euglucon), 1-(4-(2-(chloro-2-methoxybenzamido)ethyl)-benzenesulphonyl)-3-cyclo hexyl. Urea, or 1-((p-(-2-(5-chloro-O-anisamido) ethyl)phenyl)-sulphonyl-3-cyclo hexyl Urea, a sulphonyl urea derivative having melting point 169-174°C is a white or almost white crystalline orderless powder, practically without taste, insoluble in water and soluble in ethanol, methanol and in diethyl ether. It dissolves in dilute solutions of alkali-hydroxides1-2. Glibenclamide is a second-generation oral hypoglycemic agent which is more potent than those of first group3 and is used to assist in the control of mild to moderately severe type (II) Diabetes mellitus. Complexation of sulphonyl urea with lighter transition metals has been studied in detail. A persual of available literature shows that many drugs possessed modified pharmacological and toxicological properties when administered in the form of metallic complexes. Probably the most widely studied cation in this respect was Cu(II), since a host of low-moleculor weight copper complexes had been proven beneficial against several diseases such as tuberculosis, gastic ulcers and cancer4-5. However in view of the above and in continuation of our work6-11, it is interesting to have an insight into the synthesis of copper (II) complex with glibenclamide and to study various.structural aspects of the isolated complex. Here the synthesis and characterization of copper complex with glibenclamide has been described
 |
Figure 1: Structure of Glibenclamide |
Experimental
Ligand-metal ratio
(a) Pure glibenclamide (I) 1-(4-(2-(chloro-2-methoxy benzamido)ethyl)-benzene sulphonyl)-3-cyclohexyl urea, a sulphonyl urea derivative is supplied by Aventis pharma Ltd; Goa in powdered form. 0.005M drug and copper (II) chloride (CuCl2.2H2O)0.01M (Analar grade) were prepared in purified 80% ethanol. Glibenclamide (20mL) was diluted to 200ml and titrated conductometrically against cupric chloride at 27±1°C. Results were plotted in the form of a graph which indicates ligand-metal ratio as 2:1.
(b) Formation of 2:1 (L2M) ratio was also confirmed by Job’s method12 of continuous variation as modified by Turner and Anderson13 using D conductance as index property. From these value D the stability constant (logk) and free energy change (-DF) were also calculated14-20. (Table 1and 2; Fig (II))
Synthesis of Complex
The chemicals used in this synthesis were all of analytical grade. A weighed quantity of glibenclamide (2mol) was dissolved in minimum quantity of 80% ethanol. The copper (II) chloride solution was prepared by dissolving it separately in the same solvent.
Ligand solution was added slowly with constant stirring into the solution of metallic salt at room temperature maintaining the pH between 6.2 to 6.9 by adding dilute NaOH solution. On refluxing the mixture for 3hr at 80 °C on cooling, the complex separated out, which was filtered off, washed well with ethanol (60%) and finally dried in vacuum and weighed. The elemental analysis of the isolated complex was carried out using the reported methods 21-22. Copper was estimated iodometrically as cuprous thiocyanate. The IR spectrum of the ligand as well as of the complex was recorded on Perkin Elmer Spectrophotometer RX1(4000-450cm-1(CDRI Lucknow) and Electronic spectra of the ligand and isolated complex was recorded on a Lambda 25 instrument model at scan speed 480.00 nm/min. (Sadhu Vaswani College, Bairagarh, Bhopal).
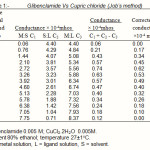 |
Table 1: Glibenclamide Vs Cupric chloride (Job’s method) |
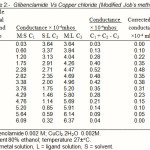 |
Table 2: Glibenclamide Vs Copper chloride (Modified Job’s method) |
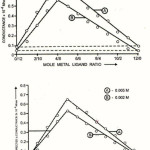 |
Figure 2: Glibenclamide Vs Copper (II ) chloride, Modified Jobs method |
Results and discussion
The synthesized complex is blue crystalline powder and stable. It is soluble in acetone, DMF and DMSO etc. and insoluble in all other organic solvents.
Infrared spectral studies
The IR spectrum of the ligand and the isolated complex was scanned in the range 4000-400cm-1 and the probable assignments are given in table4. The proposed structure for the isolated complex is also supported by IR absorption bands and characterized by the prominent band in the region of 3311-3365cm-1 due to urea NH stretching, a very sharp peak observed at 2929cm-1 due to –CH stretching. At 1713cm-1 the absorption was due to C = O, a strong absorption band at 1524cm-1 due to C = C, at 1342cm-1 due to C = O and at 1156cm-1 the peak of SO2 was observed. The absorption peak of chlorine occurred at 537cm-1. The carbonyl band was observed at 1713cm-1 in reference standard, but in transition metal complex this stretching frequency, which got shifted downward at 1709cm-1 region. Also the absorption peak of uS = O group is observed at 1151cm-1 in the complex. The shift of the uC = O and uS= O by decreasing frequency in the complex indicate that these groups are in involved in the complexation. The linkage through amide O and sulphone-O-atom was further supported by the appearance of a band in the far IR region at 671cm-1 in the complex that may be assignable to M-O frequency23-24.
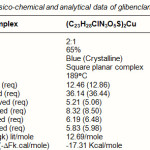 |
Table 3: Physico-chemical and analytical data of glibenclamide-copper complex |
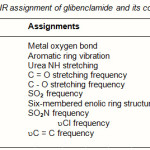 |
Table 4: Specific IR assignment of glibenclamide and its copper complex. |
b)Electronic spectral studies of complex
The electronic spectrum of Cu (II) chelate shows low intensity shoulder bands centered at 15,690 and 21,870cm-1. Under the influence of the tetragonal distortion, the 2Eg and 2T2g states of the octahedral Cu (II) ion (d9) split such a to cause the three transitions 2B1g®2B2g, 2B2g®2Eg and 2B1g®2A1g remain unresolved in the spectra25-30. An intense peak observed at 25490cm-1 is due to ligand to metal charge transfer transition.
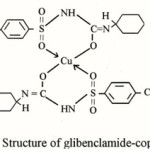 |
Figure 3 Click here to View figure |
From the present study it can be concluded that the study of chemistry and chemical reaction of co-ordination compound help in establishing structure activity relationship and it has also been observed that in biological activity metal complex is more potent and less toxic as compared to the free ligand.
Acknowledgements
the authors are thankful to principal, Sadhu VaswanI College Bairagarh for providing necessary facilities for research work
References
- Nartindale the complete drug reference, pharmaceutical press, London 32nd Ed, 320 (1999).
- Sorenson, 1976 J.R.J: Sorenson, J, Med, Chem. 19 (1976)p.135. View record in scopus/cited by in scopus (138).
- Iqbal S.A and Jacob George; Orient.J.Chem 23(3), 1123-1126 (2007).
- Dinesh Gupta and Durga Dass; Arun Syamal; Amit kumar; Orient.J.Chem; 25 (4) 871-877 (2009).
- Salil Sharma; S.A.Iqbal; and Mamta Bhattacharya; Orient.J.Chem; 25 (4) 1101-1104 (2009).
- S.A. Iqbal and G. Jacob, Orient. J. Chem. 12, 148 (1996)
- S.A. Iqbal and G. Jacob, and S. Malik, Orient. J. Chem. 14, 116 (1998).
- F. Afridi, S.A. Iqbal and G. Jacob, Orient. J. Chem. 20, 413 (2004).
- A Siddiqui, S.A. Iqbal and R. Qureshi, Orient. J. Chem. 20, (2004).
- A Siddiqui, S.A. Iqbal and S. Qureshi, Orient. J. Chem. 20, 655 (2004).
- P. Budhani, S.A. Iqbal and S. Malik, Orient. J. Chem. 21, 147 (2005).
- P. Job, Ann.clin, 113. 10 (1928)
- S.E. Turner and R.C. Anderson . J. Am. Chemsoc, 912, 71 (1949).
- S.M. Hinton and D. Dean, Crit. Rev. Microbiol., 17, 169 (1990).
- E.I. Sitefel, Prog. Inorg. Chem, 22, 1 (1977).
- H. Irving and H.S. Rossotti, J. Chem. Soc., 2904 (1954); 3397 (1953); 1176 (1995).
- H.H. Willard, L.L. Merritt Jr. and J.A. Dean, Instrumental Methods of Analysis, Van Nostrand Co, New York, edn. 5 (1974).
- G.H. Jeffery, J. Bassett, J. Mendham and R.C. Denney, Vogel’s Text Book of Quantitative Chem. Analysis, edn. 5. p. 473 (1989).
- M. Ashraf, M.A. Siddiqui and M.K. Bhatty, Talanta, 15, 559 (1989).
- A. Scott, Standard Methods of Chemical Analysis, Van Nostrand, p.634 (1960).
- S.E.I. Houte and M.E.I. Sayed Ali, J. Therm. Anal., 37, 907 (1991)
- R.C. Mackenzie, Thermochim. Acta, 73, 251 (1984). New York (1973).
- F. Szabadvary and E.B. Ghee, J. Therm. Anal., 15, 389 (1979)
- R.K. Agrawal, I. Chakraborti and N.K. Sharma, Orient. J. Chem., 19, 95 (2003).
- M.C. Patel and A.D. Shah, Orient. J. Chem., 40, 1166 (2001).
- B.D. Cullity, Elements of X-ray Diffraction, Addison Wesley Publishing Company, Inc., edn. 2 (1978)
- W.L. Bragg and W.H. Bragg, The Crystalline State, A General Survey, London, Vol. 1 (1993)
- K. Nakamotto, Infrared Spectra of Inorganic and Coordination Compounds, John Wiley & Son’s New York Ed. (1963).
- C.N.R. Rao, Chemical Applications of Infrared Spectroscopy, Academic Press, New York (1963).
- L.J. Betlamy, The Infrared Spectra of Complex Molecules, Matheun and Co. Ltd., London (1964).

This work is licensed under a Creative Commons Attribution 4.0 International License.









