Synthesis and Physico-Chemical Characterisation of New Mannich Bases and Their Paramagnetic Metal Complexes
Mohammed Rais Khan and Sahdev
Department of Chemistry, Government Raza P.G.College, Rampur India.
A new Mannich base,N-(morpholinobenzyl)benzamide (MBB),formed by the condensation of morpholine,benzamide and benzaldehyde and its Fe(II), Fe(III), Mn(III), Co(III), Ti(III), V(III), V(IV), MoO(V), Ru(II) and Ru(III) complexes have been Synthesized.Their probable structures have been determined on the basis of their microanalytical,IR,UV-vis 1H NMR spectral data. All the complexes exhibit octahedral geometry. The molar conductance data suggested 1:1 electrolytic nature for all complexes except Ru(II) and Fe(II) which are non-electrolytes and V(IV) is 1:2 electrolyte. Based on these studies all the complexes have been assigned octahexzobity. The biological activities of the ligand and its metal chelates against the bacteria E.coli,Staphylococcus aureus, Klebsiella pneumonia, Salmonella typhi, Pseudomonas aeruginosa and Shigella flexnri are also reported. The complexes have higher activity than that of the free Mannich base and the control.
KEYWORDS:N-(morpholinobenzyl) benzamide; Morpholine; Benzamide and Mannich base
Download this article as:| Copy the following to cite this article: Khan M. R, Sahdev S. Synthesis and Physico-Chemical Characterisation of New Mannich Bases and Their Paramagnetic Metal Complexes. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Khan M. R, Sahdev S. Synthesis and Physico-Chemical Characterisation of New Mannich Bases and Their Paramagnetic Metal Complexes. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=25067 |
Introduction
It is well known from the literature that the compounds containing amide moiety haνe a strong ability to form metal complexes and exhibit a wide range of biological actiνities1-4. The coordination chemistry of amide group has received much attention due to its diverse coordinating behaviour and the role it plays in biological process5. An amide group offers two potential sites, i.e., through oxygen and nitrogen for complexation with protons and metal ions. It is now generally accepted that for neutral amide groups, both protonation and metal ion binding will be at the amide oxygen6. Upon deprotonation the binding shifts to the amide nitrogen7. But, for certain reasons, like bit size and steric hindrance, the coordination may also take place at amide nitrogen.
Keeping these facts in view, an attempt has been made to synthesize and characterize the complexes of metals a new Mannich Base, N-(morpholinobenzyl) benzamide (MBB) which also contains amide moiety. The synthesis of N-(morpholinobenzyl)benzamide (MBB)and its complexation behaviour with Fe(II), Fe(III), Mn(III),Co(III),Ti(III), V(III),V(IV),MoO(V), Ru(II) and Ru(III) salts and the antibacterial activity of the complexes are described in this note.
Experimental
All the chemicals and reagents used were of AR grade or equivalent purity. Spectroscopic grade solvents were used for spectral analysis. The carbon, hydrogen, nitrogen and Chlorine content in each complex were performed at CDRI, Lucknow. The ligand as well as its corresponding metal complexes were analysed by standard methods. Conductivity measurements were carried out with Philips conductivity Bridge Model PR 9500 at room temperature and 10-3 M dilution. Magnetic susceptibility was determined by Gouy’s balance. Copper sulphate was used as the calibrant.
The IR spectra were recorded in KBr pellets using a Perkin-Elmer 783 spectrophotometer. The UV-νis spectra of the complexes were recorded on a Shimadzu UV-1601 spectrophotometer. Ti(III) was prepared by the standard procedure while the other metals used as such.
Antimicrobial Activity
The in νitro biological screening effects of the investigated compounds were tested against the bacteria, S. aureus, S. typhi, K. pneumoniae, S. flexneri, P. aeruginosa, E. coli by the well diffusion method using agar nutrient as the medium. The test solutions were prepared by dissolving the compounds in DMSO. In a typical procedure8, a well was made on the agar medium inoculated with microorganisms. The well was filled with the test solution using micropipette and the plate was incubated at 35oC for 25 h. During this period, the test solution was diffused and the growth of the inoculated micro organisms was affected. The inhibition zone developed on the plate was measured. Here ampicillin is used as the control.
Synthesis Of Mannich Base
Benzamide (1.21 g, 10 mmol) in 20 ml of ethanol was mixed with morpholine (0.9 ml, 10 mmol) with stirring to get a clear solution under ice cold condition. To the contents,benzaldehyde (1 ml, 10 mmol) was added dropwise using dropper with stirring for 15-20 min.The reaction mixture was then kept at room temperature for 5 days. The colourless solid obtained was filtered and recrystallized from ethanol
(Yield: 70%; m.p. 85 oC).
The proposed structure of the ligand.
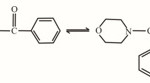 |
Figure 1 Click here to View figure |
Synthesis Of Metal Complexes
A solution of 5 mmol of metal salts and the Mannich base (10 mmol, 2.92 g) in 40 ml ethanol and chloroform mixture (1:6, ν/ν) was boiled under reflux at 58oC (b.p. of azeotropic mixture) for 3 h. The resulting solution was concentrated and then cooled to 0oC for 12 h and the precipitated complexes were filtered, washed with ethanol and dried in νacuo.
Result and Discussion
The analytical data of the synthesized and the complexes is given in table 1. The data suggested 1:2 (M:L) stiochiometry for all the complexes. The molar conductance values determined at 10-3 M dilution and 250C in both DMF and DMSO suggested 1:1 electrolytic nature for Fe(III), Mn(III),Co(III),Ti(III),V(III),V(IV),MoO(V),andRu(III)complexes where as Fe(II)and Ru(II) complex were non-electrolytic in nature. The V (IV) complex is 1:2 electrolyte.
IR spectrum of the ligand shows bands at 3380,1600 and 1100 cm-1 whichhave been assigned to ν(NH),amide ν(C=O) and ν(C-N-C) of morpholine group respeνtiνely9.In all the complexes, the ν(N-H) band appeared in the region 3330-3425 cm-1.The amide ν(C=O)and ν(C-N-C) of morpholine bands displayed substantial negative shifts with fairly low intensity indicating coordination through the oxygen of amide moiety and nitrogen of morpholine entity present in the ligand.Some new bands were found around 420-400 cm-1 and 540-520 cm-1 assignable to the MoO(V) complex showed a band at 855 cm-1assignable to Mo=O moiety. In all complexes the presence of water molecule is indicated by appearance of two non ligand bands in their IR spectra in the range of 3310–3400 cm-1 due to ν (OH) group of coordinated water molecule and 840–850 cm-1 due to wagging mode 10.
1H NMR spectra of the ligand and its Ti (III) complex were recorded in DMSO-d6 solution. The spectrum of the ligand shows the signals as follows: a broad absorption around 6.2 δ due to the NH proton; morpholine N-CH2 at 2.2 δ and morpholine O-CH2 at 3.7 δ; the methyline proton appeared in 6.7 δ; the multiplet observed around 7.2 to 8.2 δ is assignable to the phenyl group. In the ligand spectrum, one absorption peak at 10.1 δ was observed which can be assigned to N=C-OH proton. The disappearance of the peaks at 10.1 δ and 6.2 δ in the spectrum of Ti (III) complex indicates that the coordination is taking place via the dissociation of the –OH proton also shifted downfield and appeared at 2.6 δ in the complex. This is an indication of the coordination of morpholine nitrogen.
Magnetic Properties And Electronic Spectra
The magnetic moment of Fe (III) complex is 5.97 B.M., corresponding to five unpaired electrons and a high spin state of Fe (III) ion. Three bands at 11235, 21470 and 27780 cm-1 corresponding to 6A1g→ 4T1g ,6A1g→ 4T2g and 6A1g→ 4Eg transition respectively, are observed in Fe (III) complex suggesting an octahedral geometry11.
Magnetic moment for Mn(III) complex is 4.90 B.M. revealing the high spin nature of the complex, corresponding to four unpaired electrons. Electronic spectra show a strong band at 19680-20000 cm-1 which can be assigned due to ligand to metal charge transfer and a shoulder at 18000-180540 cm-1 may be assigned to the 5Eg→ 5T2g transition12, 13.
The Co (III) complex is diamagnetic (at 270C) as expected for a low spin d6 ion. The electronic spectra of Co(III) complex display band at 15110-15450,21095-21640 and 23370-23860 cm-1 . These are similar to those reported for other six coordinated Co(III) complex12-15 and may be assigned to 1A1g→3T2g , 1A1g→4T1g and 1A1g→1T2g transition respectively.
The Ti(III) complex shows magnetic moment of 1.69 B.M. for one unpaired electron. The higher value may be due to the orbital contribution. A single broad band has been observed at 19230 cm-1 for Ti(III) complex derived from the transition 2A2g→ 2Eg for an octahedral symmetry16.
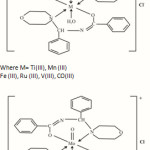 |
Figure 2 Click here to View figure |
The electronic spectrum of V(III)complex exhibited a band at 16000 cm-1 with a shoulder at 20,500 cm-1.The low energy band has been assigned to T1g→ 3T2g and the high energy band to 2T1g→ 3T1g (P) transition respectively. These bands are characterized of octahedral geometry17.The electronic spectrum of V(IV) complex exhibited a single band at 12820 cm-1.In the absence of finely resolved spectra, it was therefore, not appropriate to judge the geometry of the complex as regular octahedral18.
The electronic spectrum of MoO(V) complex suggested that the complex may be considered as octahedral with a strong tetragonal distortion resulting from Mo=O band19.The spectrum exhibited three distinct absorption bands in the ligand field region. The low intensity band at 13000 cm-1 in the long wavelength region, is possibly due to first crystal field transition 2B2→ 2E(dxy,dyz,dxz).The second crystal field transition at 19000 cm-1 is assignable to 2B2→ B1(dxy → dx2 _ y2).The third peak was observed at 30,000 cm-1 assignable to 2B2→ 2A1(dxy-dz2).
The µeff for this complex was found to be 1.88 B.M..as expected for an octahedral complex with t52g configuration. Its electronic spectrum in chloroform displayed band at 17860,21500,27780 and 33900 cm-1,which may be assign to 2A2g→ 4T1g , 2A2g→ 4T2g , 2A2g→ 2A2g, 2A1g and charge transfer respectively. These are similar to those reported to octahedral Ru (III) complex20.
The determination of magnetic susceptibility of Ru(II) complex by Gouy’s method indicated diamagnetic nature of the complex .The electronic spectrum of the complex showed a single band assignable to CT transition.
The Fe(II) complex shows magnetic moment of 5.01 B.M. and bands at 13200 and 19340 cm-1 due to d-d and CT transition respectively.
On the basis of above mentioned evidences octahedral geometry may be suggested for all the complexes with possible distortion in case of MoO(V) complex due to Mo=O moiety.
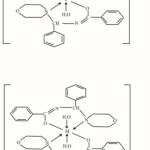 |
Figure 3 Click here to View table |
![Table 1 – Analytical data of the complexes [Found (Calcd), %]](http://www.orientjchem.org/wp-content/uploads/2011/06/Vol27_Iss2_Moh_Synl_Tab1-150x150.jpg) |
Table 1: Analytical data of the complexes [Found (Calcd), %] Click here to View table |
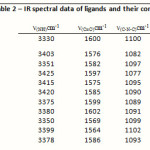 |
Table 2: IR spectral data of ligands and their complexes Click here to View table |
Acknowledgement
The Authors are thankful to the Head of Chemistry Department and Principal Government Raza P.G. College, Rampur for providing facilities to carryout this research work.
References
- Kasim A N M, Venkappayya D & Prabhu G V, J Indian Chem Soc,76 (1999) 67.
- Desai P S & Desai K R, J Indian Chem Soc, 70 (1993) 177.
- Gandhi J B & Kulkarni N D, Polyhedran, 18 (1999) 1735.
- Raman N & Ravichandran S, Asian J Chem, 15 (2003) 255.
- Seigel H & Martin R B,Chem Rev, 82 (1982) 385.
- Hondrellis V, Abanos T K,Perlepes S P & Tsangaris J M,Inorg Chim Acta, 36 (1987) 1.
- Garg B S, Reddy M J, Kumar V & Aggarwal M B, J Indian Chem. Soc, 70 (1993) 1017.
- Pelezar M J,Chan E C S & Krieg N R,Microbiology,5th Edn (New York), (1988).
- C.N. Rao & J.R. Ferraro, Spectroscopy in Inorg.Chem. Acadamic press, New York,10:149 (1970).
- Rai P K & Prasad R N, Synth react Inorg Met –Org Chem, 24 (1994) 907.
- Kolawole G A, Synth React Inorg Met –Org Chem, 23 (1993) 907.
- Patel I A, Thaker B T & Thaker P B, Indian J Chem, 37A (1998) 429.
- Lever A B P, Inorganic Electronic spectroscopy, (Elsevier, Amsterdam) 1984, pp 275-77.
- Narang K K & Singh V P, Synth React Inorg Met –Org Chem., 23 (1993) 971.
- Saha N C, Butcher R S, Chaudhuri S & saha N, Polyhedran, 21 (2002) 779.
- S R Aswale, P R mandlik, S S Aswale & A S Aswar, Indian Journal of Chemistry Vol. 42 A, Feb 2003, pp.322-326.
- D.J.Machin & K.S. Murray, J.Chem Soc.A. 1498 (1961).
- S.J. Swany, A.Dhama reddy & K Bhaskar, Indain Journal of Chem.; 40 A: 1166 (2001).
- Richa Saxena, Sahdev and Shamim Ahmad Oriental Journal of Chemistry Vol. 26 (4),1507-1511 (2010).
- A.K. Singh, B K Puri and P K Rawlley, Indian journal of Chemistry, 28 A pp.59-62 (1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.









