Synthesis and Antimicrobial Evaluation of 5-(p-substituted phenyl)-N-(3-(5-nitrofur-2-yl) allylidene)-1,3,4-thiadiazol-2-amine Derivatives
Nadia Salih¹*, Jumat Salimon¹ and Emad Yousif²
¹School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor (Malaysia). ²Department of Chemistry, College of Science, Al-Nahrain University, Baghdad (Iraq).
5-(p-Substituted phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amines 10-17 were prepared utilizing different p-substituted benzoic acid through two step reactions. Firstly, thiosemicarbzide was reacted with benzoic acid derivatives 1 in the presence of phosphorus oxychloride to give 2- amino-5-substituted-1,3,4-thiadiazoles 2-9. These compounds were reacted with 5-nitro-2-furanacrolein to yield the target products. The structures of synthesized compounds were confirmed on the basis of their elemental analysis, spectral results (IR, 1 H and 13C NMR) and mass spectra. The synthesized compounds were screened for their antimicrobial activities. The preliminary results revealed that some of the compounds exhibited promising antimicrobial activities.
KEYWORDS:Antimicrobial activity; 1,3,4-Thiadiazole; 5-Nitro-2-furanacrolein; Schiff base
Download this article as:| Copy the following to cite this article: Salih N, Salimon J, Yousif E. Synthesis and Antimicrobial Evaluation of 5-(p-substituted phenyl)-N-(3-(5-nitrofur-2-yl) allylidene)-1,3,4-thiadiazol-2-amine Derivatives. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Salih N, Salimon J, Yousif E. Synthesis and Antimicrobial Evaluation of 5-(p-substituted phenyl)-N-(3-(5-nitrofur-2-yl) allylidene)-1,3,4-thiadiazol-2-amine Derivatives. Available from: http://www.orientjchem.org/?p=11669 |
Introduction
In the past decades, the problem of multidrug resistance microorganisms has reached on alarming level around the world and the synthesis of new anti-infective compounds has become an urgent need for the treatment of microbial infections. The 1,3,4-thiadiazole nucleus has been incorporated into a wide variety of therapeutically important agents (Lamani and Kotresh, 2010). Many drugs containing thiadiazole nucleus are available in the market such as acetazolamide, methazolamide, sulfamethazole, etc. (Pandey et al., 2011). Thiadiazole is a 5-membered ring system containing two nitrogen and one sulphur atom. They occur in nature in four isomeric forms viz. 1,2,3-thiadiazole; 1,2,5-thiadiazole; 1,2,4-thiadiazole and 1,3,4-thiadiazole. The 1,3,4-thiadiazole isomer of thiadiazole series and its dihydro-derivatives provide a bulk of literature on thiadiazole.
A glance at the standard reference work shows that more work has been carried out on the 1,3,4-thiadiazole than all other isomers combined (Singh et al., 2009). Members of this ring system have found their way into many diverse applications such as pharmaceuticals (Demirbas et al., 2009), oxidation inhibitors (Mathew et al., 2011), cyanine dyes and metal complexing agents (Almasirad et al., 2007). The literature review showed that the thiadiazole nuclei have antimicrobial (Amir et al., 2009), anti-inflammatory (Hadizadeh and Vosoogh, 2008), anticancer (Ibrahim, 2009), anticonvulsant (Gilani et al., 2010), antidepressant (Yusuf et al., 2008), antioxidant (Almajan et al., 2010), radio protective (Swamy et al., 2006) and anti-leishmanial activities (Kolavi et al., 2006).
Padmavathi et al. (2008) synthesized a few 2-(aryl-methanesulfonylmethyl)-5-aryl-1,3,4- thiadiazoles which showed enhanced activity with the presence of benzylsulfonyl group and chloro substituent. On the other hand, some of the novel methylene bridged benzisoxazolyl imidaozo [2,1-b] [1,3,4] thiadiazoles derivatives were synthesized by Lamani et al. (2009) displayed very good antibacterial and/or antifungal activity. Furthermore, a number of new 5-(1H-indol-3-yl methyl)-N-(substituted phenyl)-1,2,4-thiadiazol-2-amine derivatives were synthesized and evaluated for their antibacterial and antifungal activity by Siddiqui and Alam (2009). The research study by Karegoudar et al. (2008) reports the successful synthesis and antimicrobial activity of new 1,2,4-triazolo [3,4-b][1,3,4] thiadiazoles bearing 2,3,5-trichlorophenyl moiety. The antimicrobial activity study showed moderate to good antibacterial and antifungal activities against pathogenic strains. SAR of these compounds showed that presence of 2,3,5-trichloro, -OCH3, 2,3-dichloro, 4-hydroxy-3-amido, 4-chloro, SCH3 groups attached to phenyl ring as well as pyridyl and bromopyridyl groups attached to the thiadiazole ring are responsible for good antimicrobial activity.
In view of the above mentioned findings and as continuation of our effort (Salimon et al., 2010a,b,c) to identify new candidates that may be of value in designing new, potent, selective and less toxic antimicrobial agents, we report herein the synthesis of some heterocyclic derivatives starting from different substituted carboxylic acids in order to investigate their antimicrobial activity (Fig. 1).
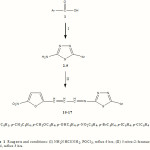 |
Table 1: Click here to View figure |
Materials and methods
Measurements
Melting points were determined in open glass capillaries on a Gallenkamp apparatus and are uncorrected. The percentage compositions of the elements (CHNS) for the compounds were determined using an elemental analyzer CHNS Model Fison EA 1108. The infrared spectra were recorded as potassium bromide discs using a Perkin-Elmer spectrophotometer GX. The 1H and 13C nuclear magnetic resonance spectra were recorded using the JEOL JNM-ECP 400 spectrometer in DMSO-d6 as solvent, using TMS as an internal standard and chemical shifts are expressed as δppm. The purities of the compounds were checked by thin layer chromatography (TLC) using ready made Silica gel plates (Merck) and benzene:methanol (8:2) as a solvent system. The spots were developed in an iodine chamber and visualized under ultraviolet (UV) lamp. Mass spectra were recorded using a Varian MTA CH-5 spectrometer (70 eV).
Synthesis
General procedure for the synthesis of 2-amino-5-substituted-1,3,4-thiadiazole 2-9
A mixture of thiosemicarbazide (0.01 mol) and aryl substituted carboxylic acid (0.01 mol), and POCl3 (5 mL) was refluxed for 4 hrs. The resultant product was transferred into a beaker to cool to room temperature then poured onto crushed ice. The solid separated out was filtered, washed with cold water and recrystalized from ethanol (Shafiee et al., 1995).
General procedure for the synthesis of 5-(p-substituted phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 10-17
A mixture of 5-nitro-2-furanacrolein (0.01 mole), appropriate 2-amino-1,3,4-thiadiazole 2–9 (0.01 mole) and 25 mL ethanol was refluxed for 3 hrs with continuous stirring. After cooling to room temperature the precipitate was filtered and recrystalized from ethanol.
5-Phenyl-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 10
M.p. 96-98 ˚C, Yield 78%. IR (KBr) υ = 3069 (C-H aromatic), 1620 (C=N), 1554 (C=C), 669 (C-S). 1H NMR (DMSO-d6) δ = 5.52-5.54 (1H, d, -CH=C-), 5.68-5.70 (1H, d, -CH=C-), 5.76-5.78 (1H, d, -CH=N-), 8.03-8.05 (d, 1H, Ar-H), 8.12-8.14 (d, 1H, Ar-H), 8.18-8.20 (d, 1H, Ar-H), 8.24-8.26 (d, 1H, Ar-H), 8.73-8.75 (t, 1H, Ar-H), 8.80-8.82 (t, 1H, Ar-H), 8.87-8.90 (t, 1H, Ar-H). 13C NMR (DMSO-d6) δ = 55.03-57.98 (3C, -C=C-C=), 63.07, 64.14 (2C, thiadiazole carbons), 87.10-89.32 (4C, furan carbons), 132.80-139.12 (6C, aromatic carbons). Elemental analysis (C15H10N4O3S); Calcd. C, 55.21; H, 3.09; N, 17.17; S, 9.83; Found C, 55.20; H, 3.10; N, 17.18; S, 9.82%; M/S: m/z (%)= 326.05 (100%), 327.05 (17.3), 328.04 (4.5%), 327.04 (1.5%).
5-(p-Methyl phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 11
M.p. 121-123 ˚C, Yield 78%. IR (KBr) υ = 3090 (C-H aromatic), 2967, 2851 (C-H aliphatic), 1623 (C=N), 1560 (C=C), 637 (C-S). 1H NMR (DMSO-d6) δ = 2.56 (s, 3H, -CH3), 5.25-5.27 (1H, d, -CH=C-), 5.34-5.36 (1H, d, -CH=C-), 5.42-5.44 (1H, d, -CH=N-), 8.49-8.51 (d, 1H, Ar-H), 8.56-8.58 (d, 1H, Ar-H), 8.63-8.65 (d, 1H, Ar-H), 8.69-8.71 (d, 1H, Ar-H), 8.75-8.77 (t, 1H, Ar-H), 8.78-8.80 (t, 1H, Ar-H). 13C NMR (DMSO-d6) δ = 56.29-58.70 (3C, -C=C-C=), 64.12, 65.38 (2C, thiadiazole carbons), 86.98-90.11 (4C, furan carbons), 131.87-138.62 (6C, aromatic carbons). Elemental analysis (C16H12N4O3S); Calcd. C, 56.46; H, 3.55; N, 16.46; S, 9.42; Found C, 56.47; H, 3.56; N, 16.47; S, 9.43%; M/S: m/z (%)= 314.07 (17.6%), 342.06 (4.8%), 341.06 (2.3%), 342.07 (2.2%).
5-(p-Methoxy phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 12
M.p. 133-135 ˚C, Yield 68%. IR (KBr) υ = 3045 (C-H aromatic), 2952, 2868 (C-H aliphatic), 1625 (C=N), 1565 (C=C), 640 (C-S). 1H NMR (DMSO-d6) δ = 3.10 (s, 3H, -OCH3), 5.22-5.24 (1H, d, -CH=C-), 5.30-5.32 (1H, d, -CH=C-), 5.36-5.38 (1H, d, -CH=N-), 8.45-8.47 (d, 1H, Ar-H), 8.51-8.53 (d, 1H, Ar-H), 8.59-8.61 (d, 1H, Ar-H), 8.64-8.66 (d, 1H, Ar-H), 8.70-8.72 (t, 1H, Ar-H), 8.74-8.76 (t, 1H, Ar-H).13C NMR (DMSO-d6) δ = 56.32-58.67 (3C, -C=C-C=), 63.07, 64.98 (2C, thiadiazole carbons), 86.67-90.59 (4C, furan carbons), 131.65-138.90 (6C, aromatic carbons). Elemental analysis (C16H12N4O4S); Calcd. C, 53.93; H, 3.39; N, 15.72; S, 9.00; Found C, 53.94; H, 3.40; N, 15.73; S, 9.01%; M/S: m/z (%)= 356.06 (100%), 357.06 (18.4%), 358.05 (4.5%), 357.05 (1.5%).
5-(p-Hydroxy phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 13
M.p. 200-202 ˚C, Yield 73%. IR (KBr) υ = 3456 (O-H), 3086 (C-H aromatic), 1623 (C=N), 1550 (C=C), 638(C-S). 1H NMR (DMSO-d6) δ = 5.31-5.33 (1H, d, -CH=C-), 5.35-5.37 (1H, d, -CH=C-), 5.40-5.42 (1H, d, -CH=N-), 8.33-8.35 (d, 1H, Ar-H), 8.40-8.42 (d, 1H, Ar-H), 8.47-8.49 (d, 1H, Ar-H), 8.55-8.57 (d, 1H, Ar-H), 8.62-8.64 (t, 1H, Ar-H), 8.68-8.70 (t, 1H, Ar-H), 9.67 (s, 1H, O-H). 13C NMR (DMSO-d6) δ = 55.92-59.07 (3C, -C=C-C=), 64.13, 66.17 (2C, thiadiazole carbons), 85.61-89.40 (4C, furan carbons), 132.05-138.12 (6C, aromatic carbons). Elemental analysis (C15H10N4O4S); Calcd. C, 52.63; H, 2.94; N, 16.37; S, 9.37; Found C, 52.62; H, 2.95; N, 16.38; S, 9.36%; M/S: m/z (%)= 342.04 (100%), 343.05 (16.5%), 344.04 (4.8%), 343.04 (2.3%), 344.05 (2.2%).
5-(p-Nitro phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 14
M.p. 169-171 ˚C, Yield 81%. IR (KBr) υ = 3065 (C-H aromatic), 1620 (C=N), 1567 (C=C), 1534, 1368 (NO2), 630 (C-S). 1H NMR (DMSO-d6) δ = 5.23-5.25 (1H, d, -CH=C-), 5.28-5.30 (1H, d, -CH=C-), 5.34-5.36 (1H, d, -CH=N-), 8.29-8.31 (d, 1H, Ar-H), 8.35-8.37 (d, 1H, Ar-H), 8.43-8.45 (d, 1H, Ar-H), 8.49-8.51 (d, 1H, Ar-H), 8.56-8.58 (t, 1H, Ar-H), 8.62-8.64 (t, 1H, Ar-H). 13C NMR (DMSO-d6) δ = 54.12-58.36 (3C, -C=C-C=), 65.52, 67.30 (2C, thiadiazole carbons), 84.07-88.15 (4C, furan carbons), 133.15-139.18 (6C, aromatic carbons). Elemental analysis (C15H9N5O5S); Calcd. C, 48.52; H, 2.44; N, 18.86; S, 8.64; Found C, 48.51; H, 2.43; N, 18.85; S, 8.65%; M/S: m/z (%)= 371.03 (100%), 372.04 (16.5%), 373.03 (4.9%), 372.03 (2.6%), 373.04 (2.4%).
5-(p-Bromo phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 15
M.p. 110-112 ˚C, Yield 83%. IR (KBr) υ = 3090 (C-H aromatic), 1627 (C=N), 1557 (C=C), 633 (C-S). 1H NMR (DMSO-d6) δ = 5.50-5.52 (1H, d, -CH=C-), 5.55-5.58 (1H, d, -CH=C-), 5.60-5.62 (1H, d, -CH=N-), 8.22-8.24 (d, 1H, Ar-H), 8.28-8.30 (d, 1H, Ar-H), 8.34-8.36 (d, 1H, Ar-H), 8.40-8.42 (d, 1H, Ar-H), 8.46-8.48 (t, 1H, Ar-H), 8.52-8.54 (t, 1H, Ar-H).13C NMR (DMSO-d6) δ = 54.30-58.16 (3C, -C=C-C=), 64.22, 68.14 (2C, thiadiazole carbons), 84.32-88.92 (4C, furan carbons), 133.25-139.38 (6C, aromatic carbons). Elemental analysis (C8H6BrN3S); Calcd. C, 37.52; H, 2.36; N, 16.41; S, 12.52; Found C, 37.53; H, 2.37; N, 16.40; S, 12.51%; M/S: m/z (%)= 403.96 (100%), 405.96 (99.5%), 406.96 (17.6), 404.96 (17.2), 405.95 (4.5%), 407.95 (4.4%), 407.96 (2.3%), 406.95 (1.5%), 404.95 (1.5%).
5-(p-Iodo phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 16
M.p. 204-206 ˚C, Yield 76%. IR (KBr) υ = 3045 (C-H aromatic), 1623 (C=N), 1550 (C=C), 631 (C-S). 1H NMR (DMSO-d6) δ = 5.41-5.43 (1H, d, -CH=C-), 5.49-5.51 (1H, d, -CH=C-), 5.55-5.57 (1H, d, -CH=N-), 8.11-8.12 (d, 1H, Ar-H), 8.18-8.20 (d, 1H, Ar-H), 8.24-8.26 (d, 1H, Ar-H), 8.29-8.31(d, 1H, Ar-H), 8.35-8.37 (t, 1H, Ar-H), 8.42-8.44 (t, 1H, Ar-H). 13C NMR (DMSO-d6) δ = 53.62-57.40 (3C, -C=C-C=), 65.81, 69.10 (2C, thiadiazole carbons), 83.15-87.37 (4C, furan carbons), 133.17-138.26 (6C, aromatic carbons). Elemental analysis (C8H6IN3S); Calcd. C, 31.70; H, 2.00; N, 13.86; S, 10.58; Found C, 31.69; H, 2.01; N, 13.87; S, 10.59%; M/S: m/z (%)= 451.94 (100%), 452.95 (16.4%), 453.94 (4.8%), 452.94 (2.3%), 453.95 (2.1%).
5-(p-Chloro phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 17
M.p. 134-136 ˚C, Yield 76%. IR (KBr) υ = 3089 (C-H aromatic), 1620 (C=N), 1556 (C=C), 633 (C-S). 1H NMR (DMSO-d6) δ = 5.41-5.43 (1H, d, -CH=C-), 5.49-5.51 (1H, d, -CH=C-), 5.55-5.57 (1H, d, -CH=N-), 8.10-8.12 (d, 1H, Ar-H), 8.11-8.12 (d, 1H, Ar-H), 8.16-8.18 (d, 1H, Ar-H), 8.22-8.24(d, 1H, Ar-H), 8.31-8.33 (t, 1H, Ar-H), 8.38-8.40 (t, 1H, Ar-H). 13C NMR (DMSO-d6) δ = 54.62-57.15 (3C, -C=C-C=), 64.80, 70.14 (2C, thiadiazole carbons), 84.10-87.27 (4C, furan carbons), 132.07-137.36 (6C, aromatic carbons). Elemental analysis (C8H6ClN3S); Calcd. C, 45.39; H, 2.86; N, 19.85; S, 15.15; Found C, 45.40; H, 2.85; N, 19.86; S, 15.16%; M/S: m/z (%)= 360.01 (100%), 362.01 (33%), 361.01 (18.7%), 363.01 (6.0%), 362.00 (4.5%), 364.00 (1.5%), 362.02 (1.3%).
Antimicrobial activity
The in vitro antimicrobial activities of synthesized compounds were carried out by cup-plate method (Barry, 1976). Antibacterial activity was screened against two gram positive bacteria (Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212) and two gram negative bacteria (Acinetobacterb baumannii ATCC 19606 and Escherichia coli ATCC 25922), whereas antifungal activity was screened against fungus Candida albicans ATCC 90028 by measuring the zone of inhibition on agar plates at two different concentrations 50 and 100 µg/mL. Ampicillin, Aztreonam and Amphotericin B were used as standard drugs. The potency of the compounds was calculated by using the following formula as described by Edwin and Marion (1945):
![]()
In the above equation, 2 is the factor for converting to percent and d is the log of the ratio of the stronger concentration to the weaker. This ratio between dilutions must be the same for both the standard and the compound being assayed. U2 is zone of inhibition of compound at 100 µg/mL, U1 is zone of inhibition of compound at 50 µg/mL, S2 is zone of inhibition of standard at 100 µg/mL, S1 is zone of inhibition of standard at 50 µg/mL.
Results and discussion
Chemistry
The designed compounds, 5-(p-substituted phenyl)-N-(3-(5-nitrofur-2-yl)allylidene)-1,3,4-thiadiazol-2-amine 10–17, were synthesized according to Fig. 1. Schiff bases under investigation were synthesized by condensation of compounds 2-9 with 5-nitro-2-furanacrolein in 1:1 molar proportion in ethanol. The reaction mixture was heated under reflux for about 3 hrs then cooled to room temperature and filtered off. The desired compounds were purified by repeated recrystalization from ethanol, dried to yield the final products. The purity of the synthesized compounds was checked by TLC, spectroscopic analysis and also by constancy of melting points.
Formation of the compounds 2-9 were confirmed by sharp bands around 3300 and 3200 cm-1 for NH2 group along with a band at about 3100 and 630 cm-1 for aromatic C-H and C-S stretching vibrations, respectively, in IR spectra (Shafiee et al., 1995, Silverstein et al., 2005). The disappearance of NH2 stretching band and the presence of sharp NO2 bands at about 1534 and 1330 cm-1 together with bands about 1625 cm-1 due to Schiff bases azomethine group (–CH=N-) stretching vibration confirmed the formation of 5-(p-substituted phenyl)-N-(3-(5-nitrofur2-yl)allylidene)-1,3,4-thiadiazol-2-amines 10-17. 1H NMR spectra showed three multiplt signals around δ 5.55-5.43 ppm assigned for –CH=CH-CH=N- and the signals at about δ 8.38-8.33 ppm was due to furan ring hydrogen’s. Furthermore, 13C NMR spectra showed three signals at about δ 53.62-57.40 ppm due –C=C-C=N- group (Sliverstien et al., 2005).
Antimicrobial activity
Most antibacterial agents used for the treatment of bacterial infections may be categorized according to their principal mechanism of action. There are four major modes of action: (1) interference with cell wall synthesis, (2) inhibition of protein synthesis, (3) interference with nucleic acid synthesis and (4) inhibition of a metabolic pathway (Neu, 1992). Antibacterial drugs that work by inhibiting bacterial cell wall synthesis include the β-lactam ring. Bacteria may manifest resistance to antibacterial drugs through a variety of mechanisms (McGowan, 2001).
Several mechanisms of antibacterial resistance are readily spread to a variety of bacterial genera. First, the bacteria organism may acquire genes encoding enzymes, such as β-lactamases, that destroy the antibacterial agent before it can have an effect. Second, bacteria may acquire efflux pumps that extrude the antibacterial agent from the cell before it can reach its target site and exert its effect. Third, bacteria may acquire several genes for a metabolic pathway which ultimately produces altered bacterial cell walls that no longer contain the binding site of the antimicrobial agent, or bacteria may acquire mutations that limit access of antimicrobial agents to the intracellular target site via down regulation of poring genes. Thus, normally susceptible populations of bacteria may become resistant to antimicrobial agents through mutation or by acquiring from other bacteria the genetic information that encodes resistance. The last event may occur through one of several genetic mechanisms, including transformation, conjugation, or transduction. Through genetic exchange mechanisms, many bacteria have become resistant to multiple classes of antibacterial agents and these bacteria with multidrug resistance (defined as resistance to ≥3 antibacterial drug classes) have become a cause for serious concern, particularly in hospitals and other health care institutions (McManus, 1997).
An antifungal drug is a medication used to treat fungal infections such as athlete’s foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis and others (Lortholary and Dupont, 1997). Antifungals work by exploiting differences between mammalian and fungal cells to kill the fungal organism without dangerous effects on the host. Unlike bacteria, both fungi and humans are eukaryotes. Thus fungal and human cells are similar at the molecular level. This makes it more difficult to find or design drugs that target fungi without affecting human cells (Fan-Havard et al., 1991). In general, fungi can be intrinsically resistant to antifungal drugs (primary resistance) or can develop resistance in response to exposure to the drug during treatment (secondary resistance).
One way to battle with this challenge is the conscious usage of the currently marketed antimicrobial agents; the other is the development of novel antimicrobial agents (Metwally et al., 2006). Hence, there will always be a vital need to discover new chemotherapeutic agents to avert the emergence of resistance and ideally shorten the duration of therapy.
In this work, the results of antibacterial activity are shown in the Table 1 and Table 2 of inhibition zones measurements at conc. 50 and 100 μg/mL. Compounds 14 has the highest activity (80.39%) while compounds 6, 7, 8, 16 and 17 exhibited good activities (51.78% – 49.88%) against gram positive bacteria S. aureus. Compounds 8 and 16 displayed highest activities (61.31% and 62.00%, respectively) against gram positive bacteria E. faecalis. On the other hand, compounds 6 and 14 have highest activities (63.90 and 75.03, respectively) while compounds 7, 8, 9, 15, 16 and 17 showed good activities (62.50% – 57.63%) against gram negative bacteria A. baumannii. Compounds 8 and 16 showed good activities (55.66% and 63.29%, respectively) against gram negative bacteria E. coli. The remaining compounds possess moderate to poor activities as compared to Ampicillin and Aztreonam.
The results of antifungal activity are shown in the Table 3 of inhibition zones measurements at conc. 50 and 100 μg/mL. Compound 16 showed the highest activity (65.25%) while the other synthesized compounds possess moderate to poor activities against C. albicans as compared to Amphotercin B.
Table 1: Gram positive antibacterial activity of compounds 2-17
|
Comp. |
Ar |
S. aureus ATCC 25923 |
Potency % |
E. faecalis ATCC 29212 |
Potency % |
||||||
|
Std: Ampicillin |
Std: Ampicillin |
||||||||||
| U2 | U1 | S2 | S1 | U2 | U1 | S2 | S1 | ||||
| 2 | C6H5 | 3 | 2 | 12 | 8 | 30.63 | 4 | 3 | 15 | 9 | 29.92 |
| 3 | p-CH3C6H4 | 2 | 1 | 12 | 7 | 25.76 | 2 | 2 | 15 | 9 | 19.22 |
| 4 | p-OCH3C6H4 | 4 | 2 | 12 | 8 | 40.83 | 4 | 3 | 15 | 9 | 27.92 |
| 5 | p-OHC6H4 | 3 | 3 | 12 | 8 | 25.29 | 3 | 2 | 15 | 9 | 25.03 |
| 6 | p-NO2C6H4 | 6 | 4 | 12 | 8 | 51.78 | 5 | 3 | 15 | 9 | 35.90 |
| 7 | p-BrC6H4 | 6 | 4 | 12 | 8 | 50.70 | 8 | 5 | 15 | 9 | 52.81 |
| 8 | p-IC6H4 | 6 | 4 | 12 | 8 | 50.88 | 9 | 5 | 15 | 9 | 61.31 |
| 9 | p-ClC6H4 | 6 | 5 | 12 | 8 | 48.20 | 8 | 5 | 15 | 9 | 54.05 |
| 10 | C6H5 | 6 | 5 | 12 | 8 | 48.22 | 6 | 4 | 16 | 9 | 39.33 |
| 11 | p-CH3C6H4 | 0 | 0 | 12 | 8 | 0 | 0 | 0 | 15 | 9 | 0 |
| 12 | p-OCH3C6H4 | 0 | 0 | 12 | 8 | 0 | 0 | 0 | 15 | 9 | 0 |
| 13 | p-OHC6H4 | 3 | 2 | 12 | 8 | 31.25 | 5 | 4 | 15 | 9 | 32.47 |
| 14 | p-NO2C6H4 | 10 | 6 | 12 | 8 | 80.39 | 3 | 3 | 15 | 9 | 20.66 |
| 15 | p-BrC6H4 | 4 | 2 | 12 | 8 | 38.83 | 3 | 3 | 15 | 9 | 22.11 |
| 16 | p-IC6H4 | 6 | 4 | 12 | 8 | 49.88 | 9 | 5 | 15 | 9 | 62.00 |
| 17 | p-ClC6H4 | 6 | 4 | 12 | 8 | 48.97 | 7 | 4 | 15 | 9 | 48.81 |
U2: Zone of inhibition of compound at 100 µg/mL; U1: Zone of inhibition of compound at 50 µg/mL; S2: Zone of inhibition of standard at 100 µg/mL; S1: Zone of inhibition of standard at 50 µg/mL.
Table 2: Gram negative antibacterial activity of compounds 2-17
|
Comp. |
Ar |
A. baumannii ATCC 19606 |
Potency % |
E. coli ATCC 25922 |
Potency % |
||||||
|
Std: Aztreonam |
Std: Aztreonam |
||||||||||
| U2 | U1 | S2 | S1 | U2 | U1 | S2 | S1 | ||||
| 2 | C6H5 | 3 | 2 | 13 | 8 | 28.41 | 3 | 2 | 14 | 9 | 27.57 |
| 3 | p-CH3C6H4 | 2 | 1 | 12 | 8 | 26.20 | 3 | 2 | 14 | 9 | 25.24 |
| 4 | p-OCH3C6H4 | 3 | 2 | 12 | 8 | 30.12 | 4 | 4 | 14 | 9 | 29.80 |
| 5 | p-OHC6H4 | 4 | 2 | 12 | 7 | 35.74 | 3 | 3 | 14 | 8 | 23.49 |
| 6 | p-NO2C6H4 | 7 | 3 | 12 | 8 | 63.90 | 5 | 3 | 14 | 9 | 37.80 |
| 7 | p-BrC6H4 | 7 | 5 | 12 | 8 | 59.10 | 6 | 5 | 14 | 9 | 40.33 |
| 8 | p-IC6H4 | 7 | 5 | 12 | 8 | 57.85 | 8 | 6 | 14 | 9 | 55.66 |
| 9 | p-ClC6H4 | 7 | 5 | 12 | 8 | 57.63 | 7 | 5 | 14 | 9 | 50.94 |
| 10 | C6H5 | 4 | 3 | 12 | 8 | 35.45 | 5 | 3 | 14 | 9 | 39.80 |
| 11 | p-CH3C6H4 | 0 | 0 | 12 | 7 | 0 | 0 | 0 | 14 | 9 | 0 |
| 12 | p-OCH3C6H4 | 0 | 0 | 12 | 8 | 0 | 0 | 0 | 15 | 9 | 0 |
| 13 | p-OHC6H4 | 5 | 2 | 12 | 8 | 48.93 | 4 | 3 | 14 | 9 | 31.78 |
| 14 | p-NO2C6H4 | 9 | 4 | 12 | 7 | 75.03 | 8 | 4 | 14 | 9 | 42.31 |
| 15 | p-BrC6H4 | 7 | 5 | 12 | 8 | 57.24 | 7 | 5 | 14 | 9 | 50.94 |
| 16 | p-IC6H4 | 8 | 5 | 13 | 8 | 62.50 | 9 | 5 | 14 | 9 | 63.29 |
| 17 | p-ClC6H4 | 7 | 5 | 12 | 8 | 59.24 | 7 | 4 | 15 | 8 | 48.03 |
U2: Zone of inhibition of compound at 100 µg/mL; U1: Zone of inhibition of compound at 50 µg/mL; S2: Zone of inhibition of standard at 100 µg/mL; S1: Zone of inhibition of standard at 50 µg/mL.
Table 3
|
Comp. |
Ar |
C. albicans ATCC 90028 |
Potency % |
|||
|
Std: Amphotericin B |
||||||
| U2 | U1 | S2 | S1 | |||
| 2 | C6H5 | 2 | 1 | 9 | 4 | 24.67 |
| 3 | p-CH3C6H4 | 0 | 0 | 9 | 4 | 0 |
| 4 | p-OCH3C6H4 | 1 | 0 | 9 | 4 | 10.94 |
| 5 | p-OHC6H4 | 2 | 1 | 9 | 4 | 24.85 |
| 6 | p-NO2C6H4 | 4 | 2 | 9 | 4 | 43.46 |
| 7 | p-BrC6H4 | 4 | 2 | 9 | 4 | 45.40 |
| 8 | p-IC6H4 | 4 | 2 | 9 | 4 | 46.23 |
| 9 | p-ClC6H4 | 3 | 1 | 9 | 4 | 36.25 |
| 10 | C6H5 | 1 | 0 | 9 | 4 | 20.75 |
| 11 | p-CH3C6H4 | 1 | 0 | 9 | 4 | 18.70 |
| 12 | p-OCH3C6H4 | 1 | 0 | 9 | 4 | 20.11 |
| 13 | p-OHC6H4 | 2 | 1 | 9 | 4 | 27.35 |
| 14 | p-NO2C6H4 | 0 | 0 | 9 | 4 | 50.41 |
| 15 | p-BrC6H4 | 2 | 1 | 9 | 4 | 24.64 |
| 16 | p-IC6H4 | 3 | 1 | 9 | 4 | 65.25 |
| 17 | p-ClC6H4 | 5 | 3 | 9 | 4 | 57.03 |
Antifungal activity of compounds 2–17
U2: Zone of inhibition of compound at 100 µg/mL; U1: Zone of inhibition of compound at 50 µg/mL; S2: Zone of inhibition of standard at 100 µg/mL; S1: Zone of inhibition of standard at 50 µg/mL.
Conclusions
The preparation procedures follow in this work offers reduction in the reaction time, operation simplicity, cleaner reaction and easy work-up. The antimicrobial data given for the compounds presented in this paper allowed us to state that the variation of antimicrobial activity may be associated with the nature of tested microorganisms and also is due to the chemical structure of the tested compounds. Performed SAR observation has showed the importance of electronic environment on antimicrobial activity. The presence of halogens (especially iodo) and nitro substituents on the aromatic ring has increased the activity of the compounds compared to those with other substituents which may be due to the presence of the versatile pharmacophore which might increase the lipophilic character of the molecules and thus facilitate the crossing through the biological membrane of the microorganisms and thereby inhibit their growth. In addition, for further investigations these findings can have a good effect on medicinal chemists to synthesize similar compounds selectively bearing iodo and nitro substituents.
Acknowledgements
We are grateful to Universiti Kebangsaan Malaysia for funding (“codes UKM-GUP-NBT-08-27-113”, “UKM-GGPM-NBT-164-2010”, “UKM-OUP-NBT-29-150/2011” and “UKM-ST-06-FRGS0113-2009”). We would like to thank the support staff from the School of Chemical Sciences and Food Technology and the faculty of Science and Technology from Universiti Kebangsaan Malaysia. Also, we offer special thanks to SRF and IIE.
References
- Almajan, G.L., Barbuceanu, S.-F., Bancescu, G., Saramet, I., Saramet, G., Draghici, C., 2010. Synthesis and antimicrobial evaluation of some fused heterocyclic [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Eur. J. Med. Chem. 45, 6139-6146.
- Almasirad, A., Vousooghi, N., Tabatabai, S.A., Kebriaeezadeh, A., Shafiee, A., 2007. Synthesis, anticonvulsant and muscle relaxant activities of substituted 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole. Acta Chim. Slov. 54, 317-324.
- Amir, M., Kumar, A., Ali, I., Khan, S.A., 2009. Synthesis of pharmaceutically important 1,3,4-thiadiazole and imidazolinone derivatives as antimicrobials. Indian J. Chem. 48B, 1288-1293.
- Barry, A.L., 1976. The antimicrobial susceptibility test, principles and practices. Philadelphia, USA.
- Demirbas, A., Sahin, D., Demirbas, N., Karaoglu, S.A., 2009. Synthesis of some new 1,3,4-thiadiazole-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activity. Eur. J. Med. Chem. 44, 2896-2903.
- Edwin, J.D., Marion, B.S., 1945. The paper-disc agar-plate for the assay of antibiotic substances. Tuckahoe, New York.
- Fan-Havard, P., Capano, D., Smith, S.M., et al. 1991. Development of resistance in Candida isolates from patients receiving prolonged antifungal therapy. Antimicrob. Agents Chemother. 35, 2302-2305.
- Gilani, S.J., Khan, S.A., Siddiqui, N., 2010. Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives of isoniazid. Bioorg. Med. Chem. Lett. 20, 4762-4765.
- Hadizadeh, F., Vosoogh, R., 2008. Synthesis of α-[5-(5-Amino-1,3,4-thiadiazol-2-yl)-2-imidazolylthio]acetic acids. J. Heterocyclic Chem. 45, 1-3.
- Ibrahim, D.A., 2009. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 44, 2776-2781.
- Karegoudar, P., Prasad, D.J., Ashok, M., Mahalinga, M., Poojary, B., Holla, B.S., 2008. Synthesis, antimicrobial and anti-inflammatory activities of some 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines bearing trichlorophenyl moiety. Eur. J. Med.Chem. 43, 808-815.
- Kolavi, G., Hegde, V., Khazi, I.A., Gadad, P. 2006. Synthesis and evaluation of antitubercular activity of imidazo[2,1-b][1,3,4]thiadiazole derivatives. Bioorg. Med. Chem. 14, 3069-3080.
- Lamani, R.S., Shetty, N.S., Kamble, R.R., Khazi, I.A., 2009. Synthesis and antimicrobial studies of novel methylene bridged benzisoxazolyl imidazo[2,1-b][1,3,4]thiadiazole derivatives. Eur. J. Med. Chem. 44, 2828-2833.
- Lamani, R.S., Kotresh, O., 2010. Synthesis and biological activity of some novel 4-(5-mercapto-1,3,4-thiadiazol-2-yl)-2-phenyl-5-[2-phenylvinyl]-2,4-dihydro-3H-1,2,4-triazol-3-one. E-Journal Chem. 7, 545-550.
- Lortholary, O., Dupont, B., 1997. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin. Microbiol. Rev. 10, 477-504.
- Mathew, B., Syresh, A.J., Mathew, G.E., Shankar, S., Kumar, S.S., 2011. Synthesis, characterization, an antimicrobial screening of some 2-amino-5-(phenyl substituted)-1,3,4-thiadiazole derivatives. Int. J. ChemTech Res. 3, 364-368.
- McGowan, J.E., 2001. Economic impact of antimicrobial resistance. Emerg. Infect. Dis. 7, 286-292.
- McManus, M.C., 1997. Mechanisms of bacterial resistance to antimicrobial agents. Am. J. Health Syst. Pharm. 54, 1420-1433.
- Metwally, K.A., Abdel-Aziz, L.M., Lashine, E.M., Husseiny, M.I., Badawy, R.H., 2006. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: synthesis and preliminary evaluation as antimicrobial agents. Bioorg. Med. Chem. 14, 8675-8682.
- Neu, H.C., 1992. The crisis in antibiotic resistance. Science. 257, 1064-1073.
- Padmavathi, V., Reddy, G.S., Padmaja, A., Kondaiah, P., Shazia, A., 2008. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 44, 2106-2112.
- Pandey, A., Dewangan, D., Verma, S., Mishra, A., Dubey, R.D., 2011. Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4- thiadiazole and its analgesic, anti-inflammatory, antibacterial and antitubercular activity. Int. J. ChemTech Res. 3, 178-184.
- Salimon, J., Salih, N., Hameed, A., Ibraheem, H., Yousif, E., 2010a. Synthesis and antibacterial activity of some new 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives. J. Appl. Sci. Res. 6, 866-870.
- Salimon, J., Salih, N., Yousif, E., Hameed, A., Ibraheem, H., 2010b. Synthesis, Characterization and Biological Activity of Schiff Bases of 2,5-Dimercapto-1,3,4-thiadiazole. Aust. J. Basic Appl. Sci. 4, 2016-2021.
- Salimon, J., Salih, N., Ibraheem, H., Yousif, E., 2010c. Synthesis of 2-N-salicylidene-5-(substituted)-1,3,4-thiadiazole as potential antimicrobial agents. Asian J. Chem. 22, 5289-5296.
- Shafiee, A., Naimi, E., Mansobi, P., Foroumadi, A., Shekari, M., 1995. Syntheses of substituted oxazolo –1,3,4-thiadiazoles, 1,3,4- oxadiazolers and 1,2,4-triazoles. J. Heterocyclic Chem., 32, 1235-1239.
- Singh, A.K., Bose, S., Singh, U.P., Shukla, S.J., Singh, V., Lohami, M., 2009. Synthesis and biological activity of some new thiadiazole derivatives. T. Pharm. Res. 2, 133-137.
- Siddiqui, N., Alam, M.S., 2009. 5-(1H-Indol-3-ylmethyl)-N-(substituted phenyl)-1, 2, 4-thiadiazol-2-amine derivatives: synthesis and biological screening. Biosci. Biotech. Res. Asia 6, 261-264.
- Sliverstien, R., Bassler, G., Morrill, T., 2005. Spectrometric identification of organic compounds. 7th ed., Inc, John-Wiley & Sons, New York.
- Swamy, S.N., Priya, B.S., Prabhuswamy, B., Doreswamy, B.H., Prasad, J.S., Rangappa, K.S., 2006. Synthesis of pharmaceutically important condensed heterocyclic 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivatives as antimicrobials. Eur. J. Med. Chem. 41, 531-538.
- Yusuf, M., Khan, R.A., Ahmed, B., 2008. Synthesis and anti-depressant activity of 5-amino-1, 3, 4-thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorg. Med. Chem. 16, 8029-8034.

This work is licensed under a Creative Commons Attribution 4.0 International License.









